- All
- Air
- Air Pollution
- Is Ontario’s air quality getting better or worse?
- Water
- Water Conditioning
- Water Facts
- Water Treatment
You may be wondering why a water softener can’t reduce TDS when it is removing minerals like calcium and magnesium.
That’s because water softeners use an ion exchange process to replace hard minerals with sodium ions.
Since sodium ions are being exchanged for calcium and magnesium ions, the TDS of your water isn’t directly affected. For every calcium and magnesium ion taken out, a sodium ion is put in. The higher the mineral content in your water, the more sodium is exchanged to soften it. The sodium content of softened water completely depends on how hard it was to begin with.
Softened water is certainly better for cleaning and bathing, and will extend the life of appliances like your washing machine and water heater. However, the spotting you notice from soft water may actually be sodium spots.
When water evaporates from your clean dishes or after washing your car, a powdery sodium residue could be left behind.
The good news is, sodium spotting can be very easily wiped off with a towel. The same cannot be said for soap scum and limescale spotting. You can also avoid sodium spots by thoroughly hand drying your car or dishes instead of letting them air dry.
WINTERIZING YOUR WATER SOFTENER – WHAT YOU NEED TO KNOW
Do you need to winterize your water softener or filter? And if so, what does winterization involve? That depends on the situation…
If you keep your water softener or filter in the basement of an occupied home, you shouldn’t have to worry about winterization. You will want to winterize your system if you have any of the following:
- A unit installed in an unheated garage
- A vacation home you’re not using in the winter
- You leave your home for an extended winter vacation
In all of these situations, it is smart to take precautions that ensure your water softener will be protected in below freezing temperatures. Frozen pipes could lead to pipes that burst, which could cause significant damage to your property as well as your water treatment equipment.
Let’s take a look at some helpful advice.
Insulation and Extra Heating
For people who plan to continue using their water softener during the winter, there are a few simple things you can do to make sure it doesn’t freeze in cold weather.
If you live in a milder climate, where the weather doesn’t get extremely cold, insulating your pipes and tanks should be enough to protect your system during the winter. You can purchase pipe insulation wrap at any home improvement store. Heat tape or electric pipe heating cables for the water lines are also a good idea.
When it comes to water softener tanks, some homeowners have an insulated box built around the system. You can also purchase plumbing insulation in sheets, or wrap an insulation blanket around them. There are even special jackets designed specifically for water softener tanks.
Because of the salt saturation, your brine tank is only likely to freeze in very cold climates where temperatures can drop below zero.
If you are using your water softener year round, the most important thing is to keep it warm enough to prevent freezing, which is why a space heater in your garage can help.
Remember, you only need to keep the temperature above 32 degrees Fahrenheit, and you should always take safety precautions as per the manufacture when using a space heater. Only turn it on when you know it is necessary.
Running water will also prevent freezing. If you’re only going to be away for a few days, you could leave a faucet running at a slow trickle to keep things moving in those pipes while you’re gone. Although not a cure-all, this will at times, prevent full-on freezing and bursting from freezing pipes.
Draining and Disconnecting a Water Softener
If you do not plan on using your water softener during the winter, and the heat in your residence will be turned off during that time, there are specific steps you should follow to disconnect, drain, and store your system.
It is recommended that you drain the tanks. This ensures there won’t be any water left in the system that could freeze and cause damage. You’ll need to put the softener into its regeneration cycle and wait until you notice the system backwashing water into the drain.
At this point, if your water softener has a manual bypass valve, it should be put into the bypass position to turn off the supply of water to the water softener. This will isolate and protect your system from the rest of the building’s supply of water during this time.
Remove the unit from the bypass valve and proceed to remove the valve from the tank. Once the riser tube is exposed, use plastic 3/8” – 1/2” plastic-tubing long enough to each the bottom of the riser tube and lower distributor. Siphoning the water from the media tank is recommended at this point.
The slower process of siphoning will ensure that all the water is removed from the tank. After water has stopped flowing from the siphon tubing, allow the tank to sit for 5 to 10 minutes. This additional time allows for all the water to completely settle to the bottom of media tank. At that time you can attempt to siphon the balance of water which has settled out.
The majority of standing water should be scooped out of the brine tank, but the solution that’s left should not freeze because of the high salt content.
Consult your user’s manual for detailed instructions on how to completely drain your tanks, or call a water treatment professional for help.
Next, unplug the water softener or turn off the switch to the power source.
You should be able to leave the brine tank in the cold, but you may want to completely remove and store your softener tank in a warmer area.
Still Have Questions? Find a Water-Right Expert!
Clear water is not always a sign of clean water, report from CNN below.
http://www.cnn.com/2017/11/09/health/cdc-water-contamination-reports/index.html
If your water is soft meaning low in hardness minerals you generally don’t have an issue with limescale deposits. However, if you have hard water (most people do) and it’s not treated you’ll see calcium carbonate deposits on your fixtures etc. So, what causes scale to deposit?
When calcium levels become supersaturated in water it can no longer be held in solution and it’s forced out, combining with bicarbonate to form calcium carbonate in its calcite state. There are two main causes of supersaturation:
- Temperature increase, the most obvious as we see scale in kettles and on heat elements.
- A pH increase, often due to a drop in pressure as carbonic acid flashes off as CO2 (think of the pssssst when you open Coke bottle). The acid reduction causes the pH to shoot up. You’ll have noticed cold water scaling at drinking water fountains.
1. Many dealers will advertise a no salt water conditioner. Any brand of water conditioner can be operated without using salt. This is done by using a salt substitute, potassium chloride. It generally costs twice as much as regular salt ( sodium chloride ), and can be difficult to find in some areas. Also, it is recommended to increase the salt setting on your control valve by about 10 % , when using a salt substitute. This is because it is not as efficient in providing the needed negative ions to the resin beads as regular salt.
2. Some companies offer catalytic filters and/or magnetic or Anti-Scale devices to “soften” your water that do not use salt, or anything else to regenerate their product. Buyer beware! If a technology had been developed that could replace a resin based water conditioner, then everyone would be selling it. I know I would. Those salt bags are heavy!
If you have a water softener now, you will be very dis-pleased with the over priced “Anti-Scale” products, should you switch.
I’ve been told that many times over the years by people who tried them.
For more details, I’d recommend this webpage written by a retired chemist. http://www.chem1.com/CQ/
And this one, http://www.uswatersystems.com/blog/2010/12/no-sal…
And this one, http://ths.gardenweb.com/forums/load/plumbing/msg…
Please do some “home work” before you waste a lot of money on something that sounds too good to be true.
*** Another customer wrote / asked:
I was tempted to switched to salt-free water conditioning system. What’s your opinion on those unit like www.nuvoh2o.com ? They claimed that you only need to replace the filter cartridge twice a year making it almost maintenance free?
***
My opinion is they don’t work… they do NOT soften the water, nor do they remove manganese or iron from the water.
Their “anti-scaling” process is very limited in it’s benefit. And anyone who has enjoyed true “conditioned” water, is always disappointed if they switch to this OVER PRICED method.
You won’t find those “testimonials” on their website.
And the Filter Cartridges are just Carbon Filters, because the media can not tolerate chlorine in the water supply, so it must be removed first.
Most Dealers use full size tanks with 1 cu.ft. of activated carbon instead of “cartridges” for better flow rate, and longer time before needing replacement of the carbon ( every 1 – 5 years depending on application ).
Millions of people take the safety of their food, water and consumer products for granted on a daily basis. Why? Because of three letters: NSF. NSF certification is your key to making sure that the products you use meet strict standards for public health protection.
Choosing a product certified by NSF lets you know the company complies with strict standards and procedures imposed by NSF. From extensive product testing and material analyses to unannounced plant inspections, every aspect of a product’s development is thoroughly evaluated before it can earn our certification.
Most importantly, NSF certification is not a one-time event, but involves regular on-site inspections of manufacturing facilities and regular re-testing of products to ensure that they continue to meet the same high standards required to maintain certification over time. If for any reason a product fails to meet one or more certification criteria, NSF will take enforcement actions to protect you, including product recall, public notification or de-certification.
Products that earn NSF certification are said to be “NSF certified” or “NSF listed” and display the applicable NSF certification mark to show that they have been tested by one of today’s most respected independent product testing organizations.
1. Hard water
1.1 What is hard water?
When water is referred to as ‘hard’ this simply means, that it contains more minerals than ordinary water. These are especially the minerals calcium and magnesium. The degree of hardness of the water increases, when more calcium and magnesium dissolves.
Magnesium and calcium are positively charged ions. Because of their presence, other positively charged ions will dissolve less easily in hard water than in water that does not contain calcium and magnesium.
This is the cause of the fact that soap doesn’t really dissolve in hard water.
1.2 Which industries attach value to hardness of water?
In many industrial applications, such as the drinking water preparation, in breweries and in sodas, but also for cooling- and boiler feed water the hardness of the water is very important.
2. Water softening
2.1 What is water softening?
When water contains a significant amount of calcium and magnesium, it is called hard water. Hard water is known to clog pipes and to complicate soap and detergent dissolving in water.
Water softening is a technique that serves the removal of the ions that cause the water to be hard, in most cases calcium and magnesium ions. Iron ions may also be removed during softening.
The best way to soften water is to use a water softener unit and connect it directly to the water supply.
2.2 What is a water softener?
A water softener is a unit that is used to soften water, by removing the minerals that cause the water to be hard.
2.3 Why is water softening applied?
Water softening is an important process, because the hardness of water in households and companies is reduced during this process.
When water is hard, it can clog pipes and soap will dissolve in it less easily. Water softening can prevent these negative effects.
Hard water causes a higher risk of lime scale deposits in household water systems. Due to this lime scale build-up, pipes are blocked and the efficiency of hot boilers and tanks is reduced. This increases the cost of domestic water heating by about fifteen to twenty percent.
Another negative effect of lime scale is that it has damaging effects on household machinery, such as laundry machines.
Water softening means expanding the life span of household machine, such as laundry machines, and the life span of pipelines. It also contributes to the improved working, and longer lifespan of solar heating systems, air conditioning units and many other water-based applications.
2.4 What does a water softener do?
Water softeners are specific ion exchangers that are designed to remove ions, which are positively charged.
Softeners mainly remove calcium (Ca2+) and magnesium (Mg2+) ions. Calcium and magnesium are often referred to as ‘hardness minerals’.
Softeners are sometimes even applied to remove iron. The softening devices are able to remove up to five milligrams per litre (5 mg/L) of dissolved iron.
Softeners can operate automatic, semi-automatic, or manual. Each type is rated on the amount of hardness it can remove before regeneration is necessary.
A water softener collects hardness minerals within its conditioning tank and from time to time flushes them away to drain.
Ion exchangers are often used for water softening. When an ion exchanger is applied for water softening, it will replace the calcium and magnesium ions in the water with other ions, for instance sodium or potassium. The exchanger ions are added to the ion exchanger reservoir as sodium and potassium salts (NaCl and KCl).
2.5 How long does a water softener last?
A good water softener will last many years. Softeners that were supplied in the 1980’s may still work, and many need little maintenance, besides filling them with salt occasionally.
3. Softening salts
3.1 Which types of salt are sold for application in a water softener?
For water softening, three types of salt are generally sold:
– Rock salt
– Solar salt
– Evaporated salt
Rock salt as a mineral occurs naturally in the ground. It is obtained from underground salt deposits by traditional mining methods. It contains between ninety-eight and ninety-nine percent sodium chloride. It has a water insolubility level of about 0.5-1.5%, being mainly calcium sulphate. Its most important component is calcium sulphate.
Solar salt as a natural product is obtained mainly through evaporation of seawater. It contains 85% sodium chloride. It has a water insolubility level of less than 0.03%. It is usually sold in crystal form. Sometimes it is also sold in pellets.
Evaporated salt is obtained through mining underground salt deposits of dissolving salt. The moisture is then evaporated, using energy from natural gas or coal. Evaporated salt contains between 99.6 and 99.99% sodium chloride.
3.2 Should we use rock salt, evaporated salt or solar salt in a water softener?
Rock salt contains a lot of matter that is not water-soluble. As a result, the softening reservoirs have to be cleaned much more regularly, when rock salt is used. Rock salt is cheaper than evaporated salt and solar salt, but reservoir cleaning may take up a lot of your time and energy.
Solar salt contains a bit more water-insoluble matter than evaporated salt. When one makes a decision about which salt to use, consideration should be given to how much salt is used, how often the softener needs cleanout, and the softener design. If salt usage is low, the products could be used alternately.
If salt usage is high, insoluble salts will build up faster when using solar salt. Additionally, the reservoir will need more frequent cleaning. In that case evaporated salt is recommended.
3.3 Is it harmful to mix different kinds of salt in a water softener?
It is generally not harmful to mix salts in a water softener, but there are types of softeners that are designed for specific water softening products. When using alternative products, these softeners will not function well.
Mixing evaporated salt with rock salt is not recommended, as this could clog the softening reservoir. It is recommended that you allow your unit to go empty of one type of salt before adding another to avoid the occurrence of any problems.
3.4 How often should one add salt to a softener?
Salt is usually added to the reservoir during regeneration of the softener. The more often a softener is regenerated, the more often salt needs to be added.
Usually water softeners are checked once a month. To guarantee a satisfactory production of soft water, the salt level should be kept at least half-full at all times.
3.5 How come water sometimes does not become softer when salt is added?
Before salt starts working in a water softener it needs a little residence time within the reservoir, since the salt is dissolving slowly. When one immediately starts regeneration after adding salt to the reservoir, the water softener may not work according to standards.
When the water softening does not take place it could also indicate softener malfunction, or a problem with the salt that is applied.
4. Softening costs
4.1 How much does a water softener cost?
Some softeners are more efficient than others and as a result the prizes may differ. There are time operated softeners and water meter-controlled softeners available. The water meter-controlled units produce the softest water per pound of salt.
Some softeners work on electricity, but some more recent water softeners use waterpower. Costs of a water softener greatly depend upon the type of water softener and the type of energy that is used, but also upon the hardness of the water that needs softening and the water use. When the water is very hard and it is used heavily, the costs of softening will rise.
Generally the costs of a water softener can vary between $ 0,20 and $ 0,40 a day.
The costs of water softeners are usually far outweighed by the benefits and cost savings obtained, through using softened water.
4.2 How much does a water softener cost during operation?
The running cost is merely the cost of salt. This is likely to be around $ 1,95 per person in the household in a month.
5. Softening drinking water
5.1 Do water-producing companies always produce softened water?
Although water-producing companies do have the opportunity to produce softened water, they will not always do so. A water producing company only has to add a water softener in its water purification system, to produce softened water cheaply.
But than consumers would not be able to have the choice to drink un-softened water.
Hard water problems are most likely to occur when water is heated. As a result, hard water causes few problems to the water supplying companies, especially when only cold water runs through their pipes.
5.2 Is softened water safe to drink?
Softened water still contains all the natural minerals that we need. It is only deprived off its calcium and magnesium contents, and some sodium is added during the softening process. That is why in most cases, softened water is perfectly safe to drink. It is advisable that softened water contains only up to 300mg/L of sodium.
In areas with very high hardness the softened water must not be used for the preparation of baby-milk, due to the high sodium content after the softening process has been carried out.
5.3 Can salt from softening installations enter drinking water?
Salt does not have the opportunity to enter drinking water through softening installations.
The only purpose of salt in a water softener is to regenerate the resin beads that take the hardness out of water.
5.4 How much sodium does one absorb from softened water?
The sodium uptake through softened water depends on the hardness of the water. Averagely, less than 3% sodium uptake comes from drinking softened water.
Estimates say that a person consumes about two to three teaspoons of salt a day, from various sources. Assuming a daily intake of five grams of sodium through food and the consumption of three quarts of water, the contribution of sodium (Na+) in the water from the home water softening process, is minimal compared to the total daily intake of many sodium-rich foods.
5.5 Will softening drinking water deprive it of essential minerals?
Softening will not deprive water of its essential minerals. Softening only deprives drinking water of minerals that cause the water to be hard, such as calcium, magnesium and iron.
6. Softeners maintenance
6.1 When does a softener resin need replacement?
When the water does not become soft enough, one should first consider problems with the salt that is used, or mechanical malfunctions of softener components. When these elements are not the cause of the unsatisfactory water softening, it may be time to replace the softener resin, or perhaps even the entire softener.
Through experience we know that most softener resins and ion exchanger resins last about twenty to twenty-five years.
6.2 Does a softener brine tank need cleaning?
Usually it is not necessary to clean out a brine tank, unless the salt product being used is high in water-insoluble matter, or there is a serious malfunction of some sort.
If there is a build-up of insoluble matter in the resin, the reservoir should be cleaned out to prevent softener malfunction.
6.3 What is ‘meshing’ and why should we avoid it?
When loosely compacted salt pellets or cube-style salt is used in a resin, it may form tiny crystals of evaporated salt, which are similar to table salt. These crystals may bond, creating a thick mass in the brine tank. This phenomenon, commonly known as ‘meshing’, may interrupt brine production. Brine production is the most important element for refreshing of the resin beads in a water softener. Without brine production, a water softener is not able produce soft water.
7. Softener operational questions
7.1 Can brine from softeners damage a septic tank?
The Water Quality Association has performed studies on this subject. These studies have indicated that a properly placed septic tank that works adequately cannot be damaged by brine that is discharged from a water softener. And softened water can sometimes even help reduce the amount of detergents discharged into a septic tank.
7.2 Can a water softener be used with lead pipes?
Lead pipe systems have to be replaced, before softened water can flow through them. Although lead pipe systems in hard water areas may not cause a problem, it is advisable to replace them anyway. When naturally or artificially softened water ends up in these lead pipe systems, it may cause the pickup of lead.
7.2 Can one measure water hardness inline?
Yes, although the measurement system is mainly applied in industrial water softeners.
8. Softening in households
8.1 Can a water softener be taken along during moving?
With modern water softeners, it is very possible to take them along during moving. Installation techniques involve quick fitting connections, similar to those used for laundry machines.
All that has to be done is closing off the inlet and outlet valves of the softener and open up the bypass valve, allowing hard water to flow to the storage tank and household taps. After that the softener can be disconnected, moved to its new location and placed there.
8.2 Can waste from a water softener be discharged directly in the garden?
As brine alters the osmotic pressure that plants rely upon to regulate water needs, direct discharge of either sodium or potassium chloride brine should be avoided.
8.3 Is softened water any help for dry skin conditions?
There are cases to be noted, in which people with dry skin conditions have benefited from water softening, because soft water is kinder to the hair and skin.
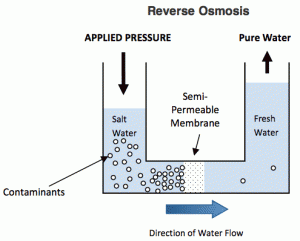
What is Reverse Osmosis?
Reverse Osmosis, commonly referred to as RO, is a process where you demineralize or deionize water by pushing it under pressure through a semi-permeable Reverse Osmosis Membrane.
Osmosis
To understand the purpose and process of Reverse Osmosis you must first understand the naturally occurring process of Osmosis.
Osmosis is a naturally occurring phenomenon and one of the most important processes in nature. It is a process where a weaker saline solution will tend to migrate to a strong saline solution. Examples of osmosis are when plant roots absorb water from the soil and our kidneys absorb water from our blood.
Below is a diagram which shows how osmosis works. A solution that is less concentrated will have a natural tendency to migrate to a solution with a higher concentration. For example, if you had a container full of water with a low salt concentration and another container full of water with a high salt concentration and they were separated by a semi-permeable membrane, then the water with the lower salt concentration would begin to migrate towards the water container with the higher salt concentration.
A semi-permeable membrane is a membrane that will allow some atoms or molecules to pass but not others. A simple example is a screen door. It allows air molecules to pass through but not pests or anything larger than the holes in the screen door. Another example is Gore-tex clothing fabric that contains an extremely thin plastic film into which billions of small pores have been cut. The pores are big enough to let water vapor through, but small enough to prevent liquid water from passing.
Reverse Osmosis is the process of Osmosis in reverse. Whereas Osmosis occurs naturally without energy required, to reverse the process of osmosis you need to apply energy to the more saline solution. A reverse osmosis membrane is a semi-permeable membrane that allows the passage of water molecules but not the majority of dissolved salts, organics, bacteria and pyrogens. However, you need to ‘push’ the water through the reverse osmosis membrane by applying pressure that is greater than the naturally occurring osmotic pressure in order to desalinate (demineralize or deionize) water in the process, allowing pure water through while holding back a majority of contaminants.
Below is a diagram outlining the process of Reverse Osmosis. When pressure is applied to the concentrated solution, the water molecules are forced through the semi-permeable membrane and the contaminants are not allowed through.
How does Reverse Osmosis work?
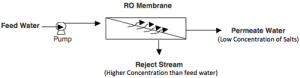
Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
The desalinated water that is demineralized or deionized, is called permeate (or product) water. The water stream that carries the concentrated contaminants that did not pass through the RO membrane is called the reject (or concentrate) stream.
As the feed water enters the RO membrane under pressure (enough pressure to overcome osmotic pressure) the water molecules pass through the semi-permeable membrane and the salts and other contaminants are not allowed to pass and are discharged through the reject stream (also known as the concentrate or brine stream), which goes to drain or can be fed back into the feed water supply in some circumstances to be recycled through the RO system to save water. The water that makes it through the RO membrane is called permeate or product water and usually has around 95% to 99% of the dissolved salts removed from it.
It is important to understand that an RO system employs cross filtration rather than standard filtration where the contaminants are collected within the filter media. With cross filtration, the solution passes through the filter, or crosses the filter, with two outlets: the filtered water goes one way and the contaminated water goes another way. To avoid build up of contaminants, cross flow filtration allows water to sweep away contaminant build up and also allow enough turbulence to keep the membrane surface clean.
What will Reverse Osmosis remove from water?
Reverse Osmosis is capable of removing up to 99%+ of the dissolved salts (ions), particles, colloids, organics, bacteria and pyrogens from the feed water (although an RO system should not be relied upon to remove 100% of bacteria and viruses). An RO membrane rejects contaminants based on their size and charge. Any contaminant that has a molecular weight greater than 200 is likely rejected by a properly running RO system (for comparison a water molecule has a MW of 18). Likewise, the greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium for example, which has two charges. Likewise, this is why an RO system does not remove gases such as CO2 very well because they are not highly ionized (charged) while in solution and have a very low molecular weight. Because an RO system does not remove gases, the permeate water can have a slightly lower than normal pH level depending on CO2 levels in the feed water as the CO2 is converted to carbonic acid.
Reverse Osmosis is very effective in treating brackish, surface and ground water for both large and small flows applications. Some examples of industries that use RO water include pharmaceutical, boiler feed water, food and beverage, metal finishing and semiconductor manufacturing to name a few.
Reverse Osmosis Performance & Design Calculations
There are a handful of calculations that are used to judge the performance of an RO system and also for design considerations. An RO system has instrumentation that displays quality, flow, pressure and sometimes other data like temperature or hours of operation. In order to accurately measure the performance of an RO system you need the following operation parameters at a minimum:
1. Feed pressure
2. Permeate pressure
3. Concentrate pressure
4. Feed conductivity
5. Permeate conductivity
6. Feed flow
7. Permeate flow
8. Temperature
Salt Rejection %
This equation tells you how effective the RO membranes are removing contaminants. It does not tell you how each individual membrane is performing, but rather how the system overall on average is performing. A well-designed RO system with properly functioning RO membranes will reject 95% to 99% of most feed water contaminants (that are of a certain size and charge). You can determine effective the RO membranes are removing contaminants by using the following equation:
The higher the salt rejection, the better the system is performing. A low salt rejection can mean that the membranes require cleaning or replacement.
Salt Passage %
This is simply the inverse of salt rejection described in the previous equation. This is the amount of salts expressed as a percentage that are passing through the RO system. The lower the salt passage, the better the system is performing. A high salt passage can mean that the membranes require cleaning or replacement.
Recovery %
Percent Recovery is the amount of water that is being ‘recovered’ as good permeate water. Another way to think of Percent Recovery is the amount of water that is not sent to drain as concentrate, but rather collected as permeate or product water. The higher the recovery % means that you are sending less water to drain as concentrate and saving more permeate water. However, if the recovery % is too high for the RO design then it can lead to larger problems due to scaling and fouling. The % Recovery for an RO system is established with the help of design software taking into consideration numerous factors such as feed water chemistry and RO pre-treatment before the RO system. Therefore, the proper % Recovery at which an RO should operate at depends on what it was designed for. By calculating the % Recovery you can quickly determine if the system is operating outside of the intended design. The calculation for % Recovery is below:
For example, if the recovery rate is 75% then this means that for every 100 gallons of feed water that enter the RO system, you are recovering 75 gallons as usable permeate water and 25 gallons are going to drain as concentrate. Industrial RO systems typically run anywhere from 50% to 85% recovery depending the feed water characteristics and other design considerations.
Concentration Factor
The concentration factor is related to the RO system recovery and is an important equation for RO system design. The more water you recover as permeate (the higher the % recovery), the more concentrated salts and contaminants you collect in the concentrate stream. This can lead to higher potential for scaling on the surface of the RO membrane when the concentration factor is too high for the system design and feed water composition.
The concept is no different than that of a boiler or cooling tower. They both have purified water exiting the system (steam) and end up leaving a concentrated solution behind. As the degree of concentration increases, the solubility limits may be exceeded and precipitate on the surface of the equipment as scale.
For example, if your feed flow is 100 gpm and your permeate flow is 75 gpm, then the recovery is (75/100) x 100 = 75%. To find the concentration factor, the formula would be 1 ÷ (1-75%) = 4.
A concentration factor of 4 means that the water going to the concentrate stream will be 4 times more concentrated than the feed water is. If the feed water in this example was 500 ppm, then the concentrate stream would be 500 x 4 = 2,000 ppm.
Flux
For example, you have the following:
The RO system is producing 75 gallons per minute (gpm) of permeate. You have 3 RO vessels and each vessel holds 6 RO membranes. Therefore you have a total of 3 x 6 = 18 membranes. The type of membrane you have in the RO system is a Dow Filmtec BW30-365. This type of RO membrane (or element) has 365 square feet of surface area.
To find the flux (Gfd):
The flux is 16 Gfd.
This means that 16 gallons of water is passed through each square foot of each RO membrane per day. This number could be good or bad depending on the type of feed water chemistry and system design. Below is a general rule of thumb for flux ranges for different source waters and can be better determined with the help of RO design software. If you had used Dow Filmtec LE-440i RO membranes in the above example, then the flux would have been 14. So it is important to factor in what type of membrane is used and to try and keep the type of membrane consistent throughout the system.
| Feed Water Source | Gfd |
| Sewage Effluent | 5-10 |
| Sea Water | 8-12 |
| Brackish Surface Water | 10-14 |
| Brackish Well Water | 14-18 |
| RO Permeate Water | 20-30 |
Mass Balance
A Mass Balance equation is used to help determine if your flow and quality instrumentation is reading properly or requires calibration. If your instrumentation is not reading correctly, then the performance data trending that you are collecting is useless.
You will need to collect the following data from an RO system to perform a Mass Balance calculation:
1. Feed Flow (gpm)
2. Permeate Flow (gpm)
3. Concentrate Flow (gpm)
4. Feed Conductivity (µS)
5. Permeate Conductivity (µS)
6. Concentrate Conductivity (µS)
The mass balance equation is:
(Feed flow1 x Feed Conductivity) = (Permeate Flow x Permeate Conductivity)
+ (Concentrate Flow*Concentrate Conductivity)
1Feed Flow equals Permeate Flow + Concentrate Flow
For example, if you collected the following data from an RO system:
| Permeate Flow | 5 gpm |
| Feed Conductivity | 500 µS |
| Permeate Conductivity | 10 µS |
| Concentrate Flow | 2 gpm |
| Concentrate Conductivity | 1200 µS |
Then the Mass Balance Equation would be:
(7 x 500) = (5 x 10) + (2*1200)
3,500 ≠ 2,450
Then find the difference
(Difference / Sum) ∗ 100
((3,500 – 2,450) / (3,500 + 2,450)) * 100
= 18%
A difference of +/- 5% is ok. A difference of +/- 5% to 10% is generally adequate. A difference of > +/- 10% is unacceptable and calibration of the RO instrumentation is required to ensure that you are collecting useful data. In the example above, the RO mass balance equation falls out of range and requires attention.
Understanding the difference between passes and stages in a Reverse Osmosis (RO) system
The term stage and pass are often mistaken for the same thing in an RO system and can be confusing terminology for an RO operator. It is important to understand the difference between a 1 and 2 stage RO and a 1 and 2 pass RO.
Difference between a 1 and 2 stage RO System
In a one stage RO system, the feed water enters the RO system as one stream and exits the RO as either concentrate or permeate water.
In a two-stage system the concentrate (or reject) from the first stage then becomes the feed water to the second stage. The permeate water is collected from the first stage is combined with permeate water from the second stage. Additional stages increase the recovery from the system.
Array
In a Reverse Osmosis System an array describes the physical arrangement of the pressure vessels in a 2 stage system. Pressure vessels contain RO membranes (usually from 1 to 6 RO membranes are in a pressure vessel). Each stage can have a certain amount of pressure vessels with RO membranes. The reject of each stage then becomes the feed stream for the next successive stage. The 2 stage RO system displayed on the previous page is a 2:1 array which means that the concentrate (or reject) of the first 2 RO vessels is fed to the next 1 vessel.
RO System with Concentrate Recycle
With an RO system that can’t be properly staged and the feed water chemistry allows for it, a concentrate recycle setup can be utilized where a portion of the concentrate stream is fed back to the feed water to the first stage to help increase the system recovery.
Single Pass RO vs Double Pass RO
Think of a pass as a stand alone RO system. With this in mind, the difference between a single pass RO system and a double pass RO system is that with a double pass RO, the permeate from the first pass becomes the feed water to the second pass (or second RO) which ends up producing a much higher quality permeate because it has essentially gone through two RO systems.
Besides producing a much higher quality permeate, a double pass system also allows the opportunity to remove carbon dioxide gas from the permeate by injecting caustic between the first and second pass. C02 is undesirable when you have mixed bed ion exchange resin beds after the RO. By adding caustic after the first pass, you increase the pH of the first pass permeate water and convert C02 to bicarbonate (HCO3-) and carbonate (CO3-2) for better rejection by the RO membranes in the second pass. This can’t be done with a single pass RO because injecting caustic and forming carbonate (CO3-2) in the presence of cations such as calcium will cause scaling of the RO membranes.
RO Pretreatment
Proper pretreatment using both mechanical and chemical treatments is critical for an RO system to prevent fouling, scaling and costly premature RO membrane failure and frequent cleaning requirements. Below is a summary of common problems an RO system experiences due to lack of proper pretreatment.
Fouling
Fouling occurs when contaminants accumulate on the membrane surface effectively plugging the membrane. There are many contaminants in municipal feed water that are naked to the human eye and harmless for human consumption, but large enough to quickly foul (or plug) an RO system. Fouling typically occurs in the front end of an RO system and results in a higher pressure drop across the RO system and a lower permeate flow. This translates into higher operating costs and eventually the need to clean or replace the RO membranes. Fouling will take place eventually to some extent given the extremely fine pore size of an RO membrane no matter how effective your pretreatment and cleaning schedule is. However, by having proper pretreatment in place, you will minimize the need to address fouling related problems on a regular basis.
Fouling can be caused by the following:
1. Particulate or colloidal mater (dirt, silt, clay, etc.)
2. Organics (humic/fulvic acids, etc)
3. Microorganisms (bacteria, etc). Bacteria present one of the most common fouling problems since RO membranes in use today cannot tolerate a disinfectant such as chlorine and therefore microorganisms are often able to thrive and multiply on the membrane surface. They may product biofilms that cover the membrane surface and result in heavy fouling.
4. Breakthrough of filter media upstream of the RO unit. GAC carbon beds and softener beds may develop an under drain leak and if there is not adequate post filtration in place the media can foul the RO system.
By performing analytical tests, you can determine if the feed water to your RO has a high potential for fouling. To prevent fouling of an RO system, mechanical filtration methods are used. The most popular methods to prevent fouling are the use of multi-media filters (MMF) or microfiltration (MF). In some cases cartridge filtration will suffice.
Scaling
As certain dissolved (inorganic) compounds become more concentrated (remember discussion on concentration factor) then scaling can occur if these compounds exceed their solubility limits and precipitate on the membrane surface as scale. The results of scaling are a higher pressure drop across the system, higher salt passage (less salt rejection), low permeate flow and lower permeate water quality. An example of a common scale that tends to form on an RO membrane is calcium carbonate (CaCO3).
Chemical Attack
Modern thin film composite membranes are not tolerant to chlorine or chloramines. Oxidizers such as chlorine will ‘burn’ holes in the membrane pores and can cause irreparable damage. The result of chemical attack on an RO membrane is a higher permeate flow and a higher salt passage (poorer quality permeate water). This is why microorganism growth on RO membranes tends to foul RO membranes so easily since there is no biocide to prevent its growth.
Mechanical Damage
Part of the pretreatment scheme should be pre and post RO system plumbing and controls. If ‘hard starts’ occur mechanical damage to the membranes can occur. Likewise, if there is too much backpressure on the RO system then mechanical damage to the RO membranes can also occur. These can be addressed by using variable frequency drive motors to start high pressure pumps for RO systems and by installing check valve(s) and/or pressure relief valves to prevent excessive back pressure on the RO unit that can cause permanent membrane damage.
Pretreatment Solutions
Below are some pretreatment solutions for RO systems that can help minimize fouling, scaling and chemical attack.
Multi Media Filtration (MMF)
A Multi-Media Filter is used to help prevent fouling of an RO system. A Multi-Media Filter typically contains three layers of media consisting of anthracite coal, sand and garnet, with a supporting layer of gravel at the bottom. These are the medias of choice because of the differences in size and density. The larger (but lighter) anthracite coal will be on top and the heavier (but smaller) garnet will remain on the bottom. The filter media arrangement allows the largest dirt particles to be removed near the top of the media bed with the smaller dirt particles being retained deeper and deeper in the media. This allows the entire bed to act as a filter allowing much longer filter run times between backwash and more efficient particulate removal.
A well-operated Multi-Media Filter can remove particulates down to 15-20 microns. A Multi-Media Filter that uses a coagulant addition (which induces tiny particles to join together to form particles large enough to be filtered) can remove particulates down to 5-10 microns. To put this in perspective, the width of a human hair is around 50 microns.
A multi media filter is suggested when the Silt Density Index (SDI) value is greater than 3 or when the turbidity is greater than 0.2 NTU. There is no exact rule, but the above guidelines should be followed to prevent premature fouling of RO membranes.
It is important to have a 5 micron cartridge filter placed directly after the MMF unit in the event that the under drains of the MMF fail. This will prevent the MMF media from damaging downstream pumps and fouling the RO system.
Microfiltration (MF)
Microfiltration (MF) is effective in removing colloidal and bacteria matter and has a pore size of only 0.1-10µm. Microfiltration is helpful in reducing the fouling potential for an RO unit. Membrane configuration can vary between manufacturers, but the “hollow fibre” type is the most commonly used. Typically, the water is pumped from the outside of the fibres, and the clean water is collected from the inside of the fibres. Microfiltration membranes used in potable water applications usually operate in “dead-end” flow. In dead-end flow, all of the water fed to the membrane is filtered through the membrane. A filter cake that must be periodically backwashed from the membrane surface forms. Recovery rates are normally greater than 90 percent on feed water sources which have fairly high quality and low turbidity feeds.
Antiscalants and Scale Inhibitors
Antiscalants and scale inhibitors, as their name suggests, are chemicals that can be added to feed water before an RO unit to help reduce the scaling potential of the feed water. Antiscalants and scale inhibitors increase the solubility limits of troublesome inorganic compounds. By increasing the solubility limits, you are able to concentrate the salts further than otherwise would be possible and therefore achieve a higher recovery rate and run at a higher concentration factor. Antiscalants and scale inhibitors work by interfering with scale formation and crystal growth. The choice of antiscalant or scale inhibitor to use and the correct dosage depends on the feed water chemistry and RO system design.
Softening by ion exchange
A water softener can be used to help prevent scaling in an RO system by exchanging scale forming ions with non scale forming ions. As with a MMF unit, it is important to have a 5 micron cartridge filter placed directly after the water softener in the event that the under drains of the softener fail.
Sodium Bisulfite (SBS) injection
By adding sodium bisulfite (SBS or SMBS), which is a reducer, to the water stream before an RO at the proper dose you can remove residual chlorine.
Granular Activated Carbon (GAC)
GAC is used for both removing organic constituents and residual disinfectants (such as chlorine and chloramines) from water. GAC media is made from coal, nutshells or wood. Activated carbon removes residual chlorine and chloramines by a chemical reaction that involves a transfer of electrons from the surface of the GAC to the residual chlorine or chloramines. The chlorine or chloramines ends up as a chloride ion that is no longer an oxidizer.
The disadvantage of using a GAC before the RO unit is that the GAC will remove chlorine quickly at the very top of the GAC bed. This will leave the remainder of the GAC bed without any biocide to kill microorganisms. A GAC bed will absorb organics throughout the bed, which is potential food for bacteria, so eventually a GAC bed can become a breeding ground for bacteria growth which can pass easily to the RO membranes. Likewise, a GAC bed can produce very small carbon fines under some circumstances that have the potential to foul an RO.
RO Data Trending and Normalization
The RO membranes are the heart of the RO system and certain data points need to be collected to determine the health of the RO membranes. These data points include the system pressures, flows, quality and temperature. Water temperature is directly proportional to pressure. As the water temperature decreases it becomes more viscous and the RO permeate flow will drop as it requires more pressure to push the water through the membrane. Likewise, when the water temperature increases the RO permeate flow will increase. As a result, performance data for an RO system must be normalized so that flow variations are not interpreted as abnormal when no problem exists. The normalized flows, pressures and salt rejection should be calculated, graphed and compared to the baseline data (when the RO was commissioned or after the membranes were cleaned or replaced) to help troubleshoot any problems and also determine when to clean or inspect the membranes for damage. Data normalization helps display the true performance of the RO membranes. As a general rule of thumb, when the normalized change is +/- 15% from the baseline data then you need to take action. If you don’t follow this rule then RO membrane cleanings may not be very effective at brining the membranes back to near new performance.
RO Membrane Cleaning
RO membranes will inevitably require periodic cleaning, anywhere from 1 to 4 times a year depending on the feed water quality. As a general rule, if the normalized pressure drop or the normalized salt passage has increased by 15%, then it is time to clean the RO membranes. If the normalized permeate flow has decreased by 15% then it is also time to clean the RO membranes. You can either clean the RO membranes in place or have them removed from the RO system and cleaned off site by a service company that specializes in this service. It has been proven that offsite membrane cleaning is more effective at providing a better cleaning than onsite cleaning skids.
RO membrane cleaning involves low and high pH cleaners to remove contaminants from the membrane. Scaling is addressed with low pH cleaners and organics, colloidal and biofouling are treated with a high pH cleaner. Cleaning RO membranes is not only about using the appropriate chemicals. There are many other factors involved such as flows, water temperature and quality, properly designed and sized cleaning skids and many other factors that an experienced service group must address in order to properly clean RO membranes.
Summary
Reverse Osmosis is an effective and proven technology to produce water that is suitable for many industrial applications that require demineralized or deionized water. Further post treatment after the RO system such as mixed bed deionization can increase the quality of the RO permeate and make it suitable for the most demanding applications. Proper pretreatment and monitoring of an RO system is crucial to preventing costly repairs and unscheduled maintenance. With the correct system design, maintenance program, and experienced service support, your RO system should provide many years of high purity water.
When water is referred to as ‘hard’ this simply means, that it contains more minerals than ordinary water. These are especially the minerals calcium and magnesium. The degree of hardness of the water increases, when more calcium and magnesium dissolves.
Magnesium and calcium are positively charged ions. Because of their presence, other positively charged ions will dissolve less easily in hard water than in water that does not contain calcium and magnesium.
This is the cause of the fact that soap doesn’t really dissolve in hard water.
Air is the Earth‘s atmosphere. It is the clear gas in which living things live and breathe. It has an indefinite shape and volume. It has no color or smell. It has mass and weight. It is a matter as it has mass and weight. Air creates atmosphere pressure. There is no air in the vacuum and cosmos.
Air is a mixture of 78.03% nitrogen, 20.99% oxygen, 0.94% argon, 0.03% carbon dioxide, 0.01% hydrogen, 0.00123%Neon, 0.0004% helium, 0.00005% krypton and 0.000006% xenon. Animals and humans need the oxygen in the air to live. In the human body, the lungs give oxygen to the blood, and give back carbon dioxide to the air. Plants need the carbon dioxide in the air to live. They give off oxygen that humans can breathe again. Wind is moving air. Air can be polluted by some gases (such as ozone and carbon monoxide), smoke, and ash. Some believe that this pollution may be one of the causes of global warming, by causing the “greenhouse effect“.
Ontario’s air quality has improved steadily since 1988. We have good air quality approximately 90 per cent of the time.
The purpose of these alerts is to inform people with breathing difficulties to avoid unnecessary exposure to smog, to inform major pollution sources that they should consider, if possible, reducing their emissions, and to solicit everyone’s help in lessening the problem by curtailing activities that produce smog.
Generally, air quality improves as you travel northward and eastward across the province, however, the formation and transport of smog is strongly dependent on meteorological conditions.
Summer smog episodes in Ontario are often a part of a regional weather condition that prevails over much of northeastern North America. Elevated levels of ozone and fine particulate matter are typically due to weather patterns that affect the lower Great Lakes region. Such weather patterns are invariably associated with slow-moving high pressure cells across the region and result in the long-range transport of smog pollutants from neighbouring U.S. industrial and urbanized states during the flow of warm air from the southwest to the northeast.
When looking for a place to live, it is important to keep local sources in mind. For instance, it is best to stay away from areas with a lot of industry and major roadways. The impacts of emissions from vehicles on a highway or any roadway depend on a number of factors, including: the distance from the highway; traffic volume; traffic congestion (i.e. free flowing or congested); and predominant wind directions.
Generally speaking, air concentration impacts from a highway decrease significantly with distance from the roadway. Typically, moving 100 metres from the edge of the road can result in a decrease in pollutant concentrations of 60-80 per cent. In addition, trees can filter the air, so a well-treed area can have better air quality than one without trees.
Smog is a general term used to describe a mixture of air pollutants, dominated by ground-level ozone and fine particulate matter. Ozone is created when nitrogen oxides and volatile organic compounds combine in the presence of sunlight – making ozone primarily a summer phenomenon, occuring mostly in southern Ontario. Fine particulate matter is primarily formed from chemical reactions in the atmosphere and through fuel combustion. It can elevate smog levels during all months of the year.
The contaminants that create smog are released during the combustion of fossil fuels in our vehicles, power plants, factory boilers and homes. They are also released by industrial processes, the evaporation of liquid fuels and the use of solvents and other volatile products such as oil-based paints. Smog-causing contaminants are released during forest fires, and emitted by natural sources such as: trees, bogs, and volcanic activity. Most of Ontario’s smog problems are caused by a combination of local emissions and pollutants carried by the wind from pollution sources in the United States. More than half of our smog problem comes from south of the border.
The Ministry of the Environment meteorologists combine real-time information on pollutant levels with data on weather patterns, topographical conditions and emission sources to predict impending smog problems. The data are obtained by a network of 40 air monitoring stations across the province. Over the last four years the ministry has invested more than $6 million in upgrading Ontario’s air monitoring network, making it the most modern and best equipped in North America.
The Air Quality Index (AQI) is an indicator of our air quality, based on pollutants that have adverse effects on human health and the environment. The pollutants are ozone, fine particulate matter, nitrogen dioxide, carbon monoxide, sulphur dioxide and total reduced sulphur compounds.
If the AQI falls below 32, the air quality is considered good or very good. An AQI reading between 32 and 49 indicates moderate air quality, and an AQI reading from 50 to 99 indicates poor air quality. A reading over 99 indicates very poor air quality.
The AQI is a calculated index that provides Ontarians with air quality information in near real-time. This index has its own concentration breakpoints for the descriptive categories (i.e. very good, good, moderate, poor, and very poor). It may be difficult to compare AQI calculations from jurisdiction to jurisdiction across North America because they are not standardized and the formulas involved are complex.
The relationship between AQI and the individual pollutant concentrations are shown below. For each category, a linear relationship is assumed between index values and the concentrations of the sub-index pollutant. At the end of each hour, the concentration of each pollutant measured at individual AQI sites is converted into a number ranging from zero upwards using a common scale or index. The calculated number for each pollutant is referred to as a sub-index. At a given site, the highest sub-index becomes the AQI reading for that hour at that location.
|
Ozone (O3) |
||
|
AQI Category |
|
AQI Equation |
| Very Good | 0 to 23 | 0.6520 × [O3] + 0 |
| Good | 24 to 50 | 0.5800 × [O3] + 2.154 |
| Moderate | 51 to 80 | 0.5900 × [O3] + 2.1 |
| Poor | 81 to 149 | 0.7200 × [O3] – 8.37 |
| Very Poor | >149 | 0.7200 × [O3] – 8.37 |
|
Fine Particulate Matter (PM2.5) |
||
|
AQI Category |
[PM2.5] 3-hour |
AQI Equation |
| Very Good | <12 | 1.364 × [PM2.5] + 0 |
| Good | 12 to 22 | 1.500 × [PM2.5] – 2.000 |
| Moderate | 23 to 45 | 0.7727 × [PM2.5] + 14.228 |
| Poor | 46 to 90 | 1.113 × [PM2.5] – 1.298 |
| Very Poor | >90 | 1.100 × [PM2.5] + 0 |
|
Sulphur Dioxide (SO2) |
||
|
AQI Category |
[SO2] (ppb) |
AQI Equation |
| Very Good | 0 to 79 | 0.1899 × [SO2] + 0 |
| Good | 80 to 169 | 0.1685 × [SO2] + 2.520 |
| Moderate | 170 to 250 | 0.2125 × [SO2] – 4.125 |
| Poor | 251 to 1999 | 0.0280 × [SO2] + 42.97 |
| Very Poor | >1999 | 0.0500 × [SO2] + 0 |
|
Nitrogen Dioxide (NO2) |
||
|
AQI Category |
[NO2] (ppb) |
AQI Equation |
| Very Good | 0 to 50 | 0.300 × [NO2] + 0 |
| Good | 51 to 110 | 0.2543 × [NO2] + 3.00 |
| Moderate | 111 to 200 | 0.191 × [NO2] + 10.79 |
| Poor | 201 to 524 | 0.1517 × [NO2] + 19.5 |
| Very Poor | >524 | 0.1903 × [NO2] + 0 |
|
Carbon Monoxide (CO) |
||
|
AQI Category |
[CO] (ppm) |
AQI Equation |
| Very Good | 0 to 12.49 | 1.2500 × [CO] + 0 |
| Good | 12.50 to 22.49 | 1.6700 × [CO] – 5.67 |
| Moderate | 22.50 to 30.49 | 2.4300 × [CO] – 23.86 |
| Poor | 30.50 to 49.49 | 2.7200 × [CO] – 34.39 |
| Very Poor | >49.49 | 2.0000 × [CO] + 0 |
|
Total Reduced Sulphur (TRS) |
||
|
AQI Category |
[TRS] (ppb) |
AQI Equation |
| Very Good | 0 to 5.49 | 3.0000 × [TRS] + 0 |
| Good | 5.50 to 10.49 | 3.7500 × [TRS] – 6.50 |
| Moderate | 10.50 to 27.49 | 1.0625 × [TRS] + 20.31 |
| Poor | 27.50 to 999.49 | 0.05046 × [TRS] + 48.59 |
| Very Poor | >999.49 | 0.1000 × [TRS] + 0 |
Notes:
- parts per billion (ppb)
- micrograms per cubic metre (µg/m3)
- parts per million (ppm)
As of May 1, 2000, as part of its new Air Quality Ontario initiative, the ministry provided earlier, more effective notification of poor air quality than ever before.
When air quality and weather conditions are likely to produce smog, the ministry takes steps to inform the public, and warn those most vulnerable to the health impacts of poor air quality. It provides two levels of alert:
- A Smog Watch is issued when there is a 50 per cent chance that elevated smog levels are forecast to occur within the next three days.
- A Smog Advisory is issued when there is a strong likelihood that elevated smog levels are forecast to occur within the next 24 hours, or it can be issued immediately if widespread, poor AQI readings occur, and weather conditions conducive to the persistence of such levels are expected to continue for several hours.
When the weather changes, resulting in cleaner air, the ministry issues an advisory termination notice.
In May 2001, the ministry adopted the policy of issuing a smog advisory immediately if widespread, poor AQI readings occur, and weather conditions conducive to the persistence of such levels are expected to continue for several hours.
As of August 2002, the ministry added fine particulate matter (PM2.5) to its AQI. This new sub-index provides Ontarians with more information on air quality so they can make informed decisions to protect their health and help improve the air we all share.
Yes. Since smog is closely tied to the weather, it is impossible to be 100 per cent accurate 100 per cent of the time. For example, a weather system could arrive in Ontario before the predicted time, or could change direction.
So poor air quality could occur without a smog advisory being called. It is also possible that poor air quality will not materialize even though a smog advisory has been called.
The Ontario Ministry of the Environment’s (MOE) Air Quality Index web site provides users with access to hourly pollutant concentration data from MOE’s ambient sites.The data output, which includes station and pollutant information, is available in both .HTML and/or .CSV format(s) which may be imported directly into Excel or any other spreadsheet application. Currently, online data is available from 2000 to 2012. The tool may be accessed at:http://www.airqualityontario.com/history/
The new SHARP monitor is able to detect additional components of PM2.5, especially during cold weather. As a result of this improvement in monitoring technology, there is potential of reporting higher PM2.5 concentrations during the winter months. This is a reflection of more accurate measurements and does not necessarily mean that Ontario’s air quality is changing. The air is the same; only the monitoring method has changed.
Ontario strives to be a leader in air quality reporting and continues to benefit from one of the most comprehensive air monitoring systems in North America. The network upgrade was funded by Environment Canada under a national initiative to standardize PM2.5 monitoring methods across Canada and ensure data comparability. The objective is to have all jurisdictions operating federally approved PM2.5 monitors by 2013.
- On this website: http://www.airqualityontario.com. This site provides Air Quality Index (AQI) readings, ambient air pollution data and air quality forecasts, as well as information on actions that can be taken when a smog alert is issued. You can also subscribe to the ministry’s smog alert network, and receive an automatic email whenever the ministry issues a smog watch or smog advisory.
- By telephone: You can get AQI readings from recorded telephone messages by dialing 1-800-387-7768 (toll-free) or 416-246-0411 in Toronto. To obtain AQI readings in French, dial 1-800-221-8852.
- Via radio and television: The MOE has worked for many years with news media across Ontario to inform the public about smog conditions. This will continue to be a crucial method for communicating information about smog and actions that can be taken to reduce smog-causing emissions. This smog season you can expect your local radio and television stations to add smog watches and smog advisories to their weather forecasts.
In 2002, Ontario was the first province in Canada to introduce monitoring of PM2.5 to the Air Quality Index. The ministry reported real-time PM2.5 with the Thermo Scientific TEOM 1400AB/SES until December 31, 2012. Continuous PM2.5 monitoring technologies have evolved dramatically over the last decade. The network is now reporting real-time PM2.5 concentrations using Thermo Scientific SHARP 5030, an approved Class III Federal Equivalent Method designated by the United States Environmental Protection Agency in 2009. Ontario evaluated the SHARP monitor starting in 2009, and adopted this method in 2012 in concurrence with Environment Canada. In 2013, the SHARP monitors were deployed across Ontario’s AQI network.
Here are some actions you can take to help protect the environment and your own health:
At home:
- Conserve electricity year-round by adjusting the heat or air conditioner and turning off lights you are not using.
- Avoid letting your car, or any other engine, idle for long periods.
- Reduce your use of gasoline-powered equipment.
- Avoid mowing the lawn when air quality is poor.
- Don’t use oil-based products such as paints, solvents or cleaners if you can avoid them. They contain volatile organic compounds (VOCs), which contribute to smog.
- Avoid or reduce strenuous physical outdoor activities when smog levels are high, especially during the late afternoon. Do not exert yourself outdoors.
- If possible, stay indoors in a cool, air-conditioned environment.
- Get engine tune-ups and car maintenance checks as advised by the car manufacturer’s maintenance schedule.
- Limit the amount of wood you burn in your fireplace or woodstove. When burning wood, use only the dry, seasoned variety.
At work:
- If possible, take public transit, or walk to work.
- If you use a car, don’t travel alone; encourage and facilitate car pooling.
- Avoid traffic congestion.
- Consider teleconferencing, instead of traveling to meetings.
As always, consult your doctor for specific medical advice on how to cope with poor air quality.
For information on smog and associated health impacts, please refer to our Smog and Your Health Fact Sheets, located at http://www.airqualityontario.com/press/publications.php.
When smog levels reach critical levels, steps should be taken immediately to protect the most vulnerable members of society and reduce emissions that could worsen local air quality. A smog alert response plan is a written protocol of actions to be taken by an organization, government agency, company or municipality when poor air quality is forecast. Such a plan should include a mechanism for informing residents or employees of the potential health threat, as well as specific actions that can be taken to reduce the risk.
More information on developing a smog alert response plan and additional suggestions for what you can do to combat smog is available in the ministry publication – Smog Alert Response: A Municipal Guide to Action.
The ministry monitors air pollution levels and issues smog advisories when there is a strong likelihood that widespread elevated and persistent smog levels are expected.
Here is a summary of smog advisories issued for Ontario by the ministry since 2003:
|
Smog advisories issued for Ontario by the ministry since 2003 |
||
|
Year |
Number of Advisories |
Total Number of Days |
| 2003 | 7 | 19 |
| 2004 | 8 | 20 |
| 2005 | 15 | 53 |
| 2006 | 6 | 17 |
| 2007 | 13 | 39 |
| 2008 | 8 | 17 |
| 2009 | 3 | 5 |
| 2010 | 3 | 12 |
| 2011 | 5 | 9 |
| 2012 | 12 | 30 |
| 2013 | 1 | 2 |
| 2014* | 0 | 0 |
* as of January 16, 2014.
Ontario is committed to doing its part to reduce emissions and improve air quality. The Air Quality Ontario initiative is one component of a comprehensive strategy to protect the environment and safeguard public health. Other components of the strategy include:
- Drive Clean – a program that reduces nitrogen oxides (NOX) and volatile organic compounds (VOCs) through emissions testing of motor vehicles and enforcement through the Smog Patrol;
- Ontario Regulation 194/05 – Industry Emissions: Nitrogen Oxides (NOX) and Sulphur Dioxides (SO2) – this regulation establishes industry sector emission caps starting in 2006 and defines how these caps will be reduced in future years (2007, 2010 and 2015);
- Ontario Regulation 419/05: Air Pollution – Local Air Quality – sets air quality standards for toxic substances to protect local communities;
- Ontario Regulation 397/01: Emissions Trading – caps nitrogen oxides (NOX) and sulphur dioxide (SO2) emissions from the electricity sector;
- Ontario Regulation 127/01: Airborne Contaminant Discharge – Monitoring and Reporting – focuses public attention on environmental practices, thus compelling high emitters to clean up their act, and OnAir: Ontario’s online registry for reporting to the public;
- Developing cleaner sources of energy to replace coal-fired generation by early 2009, a mandate to create renewable energy, encouraging energy efficiency and conservation programs, including use of co-generation;
- Stage 1 Vapour Recovery (Ontario Regulation 455/94) requires gasoline facility operators to install, maintain and operate gasoline vapour recovery systems, and Gasoline Volatility (Ontario Regulation 271/91 as amended by Ontario Regulation 45/97) which limits gasoline vapour pressure during the summer;
- Environmental Training for Dry Cleaners (Ontario Regulation 323/94);
- Canadian Council of Ministers of the Environment (CCME) Guideline A-5 for New and Modified Combustion Turbines; and,
- CCME Guideline A-9 for New Commercial/Industrial Boilers and Heaters.
Particulate matter (PM) consists of airborne particles in solid or liquid form. PM may be classified as primary or secondary, depending on the compounds and processes involved during its formation. Primary PM is emitted at the emissions source in particle form, for example, the smokestack of an electrical power plant or a recently tilled field subject to wind erosion. Secondary PM formation results from a series of chemical and physical reactions involving different precursor gases, such as sulphur oxides and nitrogen oxides, and ammonia reacting to form sulphate, nitrate and ammonium particulate matter.
The size of PM particles largely determines the extent of environmental and health damage caused. For this reason, Environment Canada identifies different sizes of PM:
Total Particulate Matter (TPM) –airborne particulate matter with an upper size limit of approximately 100 micro metre (µm) in aerodynamic equivalent diameter
Particulate Matter <10 microns (PM10) – airborne particulate matter with a mass median diameter less than 10 µm
Particulate Matter < 2.5 microns (PM2.5) – airborne particulate matter with a mass median diameter less than 2.5 µm
Numerous studies have linked PM to aggravated cardiac and respiratory diseases such as asthma, bronchitis and emphysema and to various forms of heart disease. PM can also have adverse effects on vegetation and structures, and contributes to visibility deterioration and regional haze.
Background
Fine particulate matter (PM2.5) is a general term for all small particles found in air measuring equal to or less than 2.5 μm in aerodynamic diameter. It is a complex mixture whose constituents vary in size, shape, density, surface area, and chemical composition (Health Canada and Environment Canada 1999; US EPA 2009). In 1987, Health Canada published Exposure Guidelines for Residential Indoor Air Quality, which set maximum acceptable long- and short-term exposure ranges for PM2.5 in homes. These guidelines are being revised to reflect the most up-to-date science on the health effects and residential exposure for PM2.5.
Exposure
Indoor PM2.5 is composed of indoor-generated PM2.5 from sources such as smoking, cooking, and cleaning, and PM2.5 that has infiltrated from the outside. In studies conducted by Health Canada in different Canadian cities, average indoor PM2.5concentrations were less than 15 µg/m3 in homes without smokers, and less than 35 µg/m3 in homes with smokers (Health Canada 2010). In general, indoor PM2.5 levels were lower than outdoor concentrations measured directly outside the home, except in homes with smokers.
Health Effects
Outdoor PM2.5, as measured at area monitoring stations, has been shown in a large number of studies to be strongly associated with cardiovascular and respiratory mortality and morbidity endpoints (Health Canada and Environment Canada 1999; WHO 2005; US EPA 2009). There is no recognized threshold of health effects for outdoor PM2.5regardless of where exposure occurs (i.e., indoors or outdoors), and there is evidence that adverse health effects occur at current levels of exposure.
A much smaller number of studies have investigated the relationship between indoor PM2.5 and health. There is some evidence for a relationship between indoor PM2.5 levels and declines in lung function and increases in exhaled nitric oxide, a marker of airway inflammation, in asthmatic children (Koenig et al. 2003; Delfino et al. 2004; Koenig et al. 2005; Trenga et al. 2006). However, changes in exhaled nitric oxide were more strongly associated with outdoor PM2.5 than indoor PM2.5 (Koenig et al. 2003; Koenig et al. 2005; Allen et al. 2008). Associations between indoor PM2.5 and subtle changes in markers of cardiovascular disease have also been observed in older adults (Delfino et al. 2008; Liu et al. 2009; Allen et al. 2011).
Guidance
The acceptable long- and short-term exposure ranges established in the 1987 exposure guidelines should be rescinded and replaced with this new guidance focusing on indoor source control to minimize long-term exposure to PM2.5 indoors.
Indoor levels of PM2.5 should be kept as low as possible, as there is no apparent threshold for the health effects of PM2.5. It is impossible to entirely eliminate PM2.5indoors, as among its sources are essential and everyday activities, such as cooking and cleaning, as well as infiltration from outdoor sources, over which residents have little or no control. However, any reduction in PM2.5 would be expected to result in health benefits, especially for sensitive individuals, such as those with underlying health conditions, the elderly or children.
The focus should be on reducing indoor sources over which homeowners and residents have some degree of control. The main recommended strategies to reduce exposure to the major sources of indoor-generated PM2.5 are:
- Cessation of smoking
- Use of a stove top fan while cooking
Other actions to reduce indoor PM2.5 levels include ensuring there is adequate ventilation, especially when doing activities that may generate PM2.5. The potential benefits of ventilation, however, may be reduced or eliminated, if outdoor PM2.5 levels are high. There is also evidence that some in-duct air filters or portable air cleaners with filters may help reduce indoor PM2.5 levels. Filter efficiency, however, is highly variable among products and the effectiveness of filters as a method to reduce indoor PM2.5 will depend on the product used and how it is maintained. A discussion of how to properly select and maintain air filters and portable air cleaners is beyond the scope of this document.
The above recommendations are consistent with Health Canada guidance to homeowners to focus on identifying the potential sources of contaminants in the home, and then on improving air quality through source control, improved ventilation and other remedial measures such as air filtration. Identification of potential sources is, in most situations, more informative and cost-effective than indoor air quality testing and comparison of measured values to quantitative guideline values.
Quantitative residential indoor air guidelines may be of use to public health and building professionals for the interpretation of results of indoor air quality studies and for the development of performance standards. With respect to indoor PM2.5, Health Canada is not proposing a specific maximum exposure limit, but is recommending that indoor PM2.5, at a minimum, be lower than PM2.5 outside the home. Having an indoor level that is greater than the outdoor level indicates a strong indoor source(s) of PM2.5 that needs to be addressed. The ratio of indoor to residential outdoor PM2.5 levels can therefore serve to highlight situations where strategies to reduce indoor-generated PM2.5 are necessary and will be most effective. The recommended PM2.5 reduction strategies can be employed in all homes. However, for those homes with a ratio of indoor to outdoor PM2.5 levels greater than one, targeted efforts to identify and remove indoor sources of PM2.5 are a priority.
Air Cleaner and Air Purifier?
When looking for a way to clean the air inside a home, it’s easy to become confused about terminology. For example, many people think that air purifiers are different than air cleaners. In reality, they are the same. The terms air purifier and air cleaner are completely interchangeable. By understanding what air purifiers are and how they work, it’s easier to zero in on the right one.
Indoor Air Pollutants and Air Purifiers
Before looking for an indoor air purifier, or air cleaner, it helps to have a basic understanding of the types of air pollutants that are often found in homes. This knowledge makes it easier to find air cleaners and air purifiers that effectively clean the air. With a basic understanding of indoor air pollutants, it is easier to make sense of the descriptions of today’s most popular air cleaners.
Volatile Organic Compounds
Volatile organic compounds, or VOCs, are gases or vapors that are emitted by many everyday household items. They may be emitted by things like new carpeting, paints, adhesives, varnishes, glues, and disinfectants. To determine whether a household product contains VOCs, look for ingredients like formaldehyde, toluene, chloride, ethylene, and benzene. Some air cleaners are designed to filter VOCs from the air, but others aren’t capable of doing so. If this is an important factor, look for air purifiers with filters that pull VOCs out of the air.
Odors and Gases
Some air cleaners can largely eliminate odors and gases from the air in a home. They do so with activated carbon filters, which are included on just about every air purifier and air cleaner that’s on the market today. These filters use a process called adsorption to force these gases and odors to become attached using a chemical reaction. Common gases and odors include aerosols, tobacco smokes, cooking odors, indoor pesticides, kitty litter, and toxins.
Airborne Particles
Most people buy air purifiers and air cleaners to eliminate airborne particles from their homes. Airborne particles include things like dust mites, pet dander, pollen, mold, plant spores, and fungi. These types of particles exacerbate conditions like asthma and allergies, so it is smart to eliminate from the air inside a home. HEPA filters are considered to be the most effective in eliminating these particles, which can vary in size considerably but are all microscopic.
The size of the average airborne particle is measured in microns. One micron equals 1/25,000 of an inch. To be classified as a HEPA filter, a filter must be able to pull 99.97 percent of airborne particles that measure as small as 0.3 microns.
| Particle Type | Size |
| Smoke | 0.003 microns to 0.04 microns |
| Bacteria | 0.3 microns to three microns |
| Fungi | 0.5 microns to five microns |
| Pet dander | 0.3 microns to 100 microns |
| Mold | Two microns to 20 microns |
| Pollen | 10 microns to 100 microns |
| Dust mite | 10 microns to 40 microns |
| Plant spore | 10 microns to 70 microns |
Microorganisms
Some air purifiers are designed to pull microorganisms from the air. In some cases, air cleaners also kill microorganisms using UV rays and other techniques. Bacteria, viruses, pathogens, and antigens are all microorganisms. Mold falls into this category as well.
Types of Air Filters
Whether it’s called an air purifier or an air cleaner, the most important components of this type of machine are its air filters. Indeed, most air purifiers and air cleaners contain at least two air filters. Some use three or four different air filters. One of the easiest ways to find the right air purifier is by looking for models that have specific types of filters. To do that, learn about the main characteristics of today’s most popular air filters.
Activated Carbon Filters
This type of filter isn’t generally used alone. Instead, it’s often used in conjunction with HEPA filters and other air filters. Activated carbon uses a chemical reaction called adsorption to pull odors, gases, and vapors from the air. The carbon that is found in these filters is treated with oxygen, which forces millions of small openings to develop. With an increased surface area, an activated carbon filter can pull odors, gases, and vapors from the air for a very long time. Some activated carbon filters can also pull VOCs from the air. They contain an extra chemical, which is known as a chemisorbent, which traps them in the filter or makes them harmless.
Ion and Ozone Generators
Although they aren’t technically filters, ion and ozone generators are designed to do the same thing: remove impurities from the air. However, instead of filtering them out, they just make them cling to surfaces around a room. These generators also often release ozone as a byproduct. This can be problematic because ozone is considered to be a lung irritant.
Electrostatic Precipitators
The same process that is used with ozone and ion generators is used with electrostatic precipitators. The primary difference is that electrostatic precipitators actually filter impurities out of the air too. However, they can also product ozone as a byproduct, so air purifiers that use this technology may not be suitable for people with respiratory conditions. The impurities are collected on plates that can be washed, so there’s no need to buy replacement filters.
Charged Media Filters
The description of these filters is almost identical to those of electrostatic precipitators. The main difference is that these filters actually use filters. They don’t use plates. As a result, it is necessary to change the filter from time to time. One of the best things about these filters is that they can trap extremely microscopic particles. Like electrostatic precipitators and ion and ozone generators, however, they also produce ozone.
Pre-Filters
If large particles are allowed to make contact with sensitive filters, air purifiers and air cleaners won’t work as effectively or as efficiently. That is why the vast majority of these machines have pre-filters as well. A pre-filter’s job is to pull larger particles from the air to keep them from being passed along to HEPA filters and other types of filters. They are typically made out of woven nylon or foam. In most cases, they can be cleaned again and again.
Antibacterial and Germicidal Filters
The only way to eliminate a huge percentage of germs and bacteria from the air is by purchasing an air purifier that includes antibacterial or germicidal filters. Sometimes, this technology involves the use of UV rays. Other times, HEPA filters are treated with specialized agents that kill bacteria, germs, and other microorganisms.
HEPA Filters
To successfully pull at least 99.97 percent of particles from the air, it’s crucial to buy an air purifier that uses HEPA filters. High-efficiency particulate air filters, or HEPA filters, were originally designed to remove radioactive dust particles from the air. However, all HEPA filters are not created equal. The higher the area is, the more effective a HEPA filter is. Look at the square footage of the filter before buying an air purifier that uses HEPA technology.
Types of Air Cleaners
There are three main air cleaners, or air purifiers, available: whole-house air cleaners, whole-house air filters, and portable air purifiers.
Whole-House Air Cleaners
Whole-house air cleaners are installed directly onto a home’s heating and cooling system. However, this is not a job for an average homeowner. A professional must install this type of system. Also, a home has to have forced-air ducts in order to make one of these systems work. It should also be noted that whole-house air purifiers often need to be wired directly into a home’s electrical system.
Whole-House Air Filters
An alternative way to clean a lot of the air in a home at one time is by using a whole-house air filter, which is designed to replace a standard furnace filter. This option is somewhat effective, but it doesn’t eliminate nearly as many impurities as other types of air cleaners.
Portable Air Purifiers
Although one portable air purifier is only going to clean the air in one room, it is going to do so in a very effective way. There are many affordable room air purifiers out there, so many people place on in each room.
Pros and Cons of Different Air Purifiers
| Type of Air Purifier or Air Cleaner | Pros | Cons |
| Whole-house air cleaner | Filters air throughout the entire home
Many options available |
Expensive
Has to be professionally installed May have to be wired into home’s electrical system May not be as efficient as portable models |
| Whole-house air filter | Convenient
Easy to use Inexpensive |
Not very effective at purifying the air in a home
Filters must be compatible with furnace |
| Portable air purifier | Lightweight
Compact May include casters or handles Huge variety of options available Options for every budget |
Only cleans the air in a single room
Quality varies a lot from one model to the next |
Air Purifier Features
Without understanding the main features of today’s air purifiers and air cleaners, finding the right option is extremely difficult.
Energy Efficiency
The energy efficiency of an air purifier can be gauged by considering how many watts of power it uses. If wattage information is unavailable, simply multiply the number of volts by the number of amps. Options range from air cleaners that operate on around 50 watts to models that run on 200 watts or more when on the highest setting.
ACH
The air changes per hour, or ACH, of an air purifier is an important characteristic. It reflects the number of times in an hour that an air cleaner exchanges the air in a room. The ACH is usually listed next to the room size. Stick with air purifiers with ACH ratings of four or higher because they exchange the air at least four times per hour.
CADR
Consider an air purifier’s clean air delivery rate, or CADR, before buying it as well. This rating is assigned by the Association of Home Appliance Manufacturers, or AHAM. The higher the CADR is, the more effective an air cleaner is at purifying the air.
Filter Replacements
Prior to purchasing an air cleaner, check to see how its filter or filters need to be changed. Also find out the typical cost to replace each filter. Without doing this research, it’s possible to spend hundreds of extra dollars per year on filters for an air purifier, which can ruin what looked like an otherwise good deal.
Conclusion
Air purifiers and air cleaners are the same, but there are many options available within this category. To find the right air cleaner or air purifier, learn more about how they work and about the types of air filters that they use.
Public health experts estimate that air pollution is responsible for 16,000 premature deaths in this country each year; at this rate, forty Canadians die from air pollution-related causes each and every day.
- Indoor air quality (IAQ) : People working indoors often experience symptoms such as headaches, shortness of breath, coughing or nausea just to mention a few. However, it is rarely possible to prove that these symptoms are related to a particular indoor air contaminant. In fact, building occupants are simultaneously exposed to a wide range of indoor air contaminants.
- Causes of IAQ problems: Indoor environment – inadequate temperature, humidity, lighting, excessive noise Indoor air contaminants – chemicals, dusts, moulds or fungi, bacteria, gases, vapours, odours Insufficient outdoor air intake
- Our lung health— and our health in general— depends on clean air. Indoor and outdoor air pollution can cause health problems, especially for people who have lung diseases.
- Outdoor air quality is an important health concern. Air pollution continues to be a serious threat to the quality of the air we breathe.
Air pollution can irritate, inflame, or destroy lung tissue. It weakens the lung’s defenses against contaminants. Even low levels of air pollution can cause health problems.
- Irritation in your eyes, nose and throat
- Wheezing, coughing and breathing difficulties
- Tightness in your chest
- Worsening of existing lung disease (asthma, COPD, etc.) and heart problems
Some people can notice symptoms from air pollution right away. For example, a person with asthma might find it hard to breathe after 10 minutes outside on a smoggy day. For other people, it may take longer to notice the health effects of outdoor air pollution. Even if you can’t feel a problem right away, it’s still possible that exposure to air pollution is hurting your health over the long term.
- children
- seniors
- people with lung disease, including asthma and COPD
- people with heart disease
- people with suppressed immune systems
Even healthy people can have breathing problems on days when there’s air pollution.
Your body has a natural cleaning and repair system that helps keep larger particles from hurting your lungs. There is a thin layer of sticky mucous and thousands of tiny hairs (cilia) lining the insides of the breathing tubes in your lungs. If you breathe in germs or particles, the mucous traps the little bits of dirt, and the cilia move together like a wave to push the dirt-filled mucous out of your lungs. Then you cough, spit up, or swallow the mucous, and the dirt is out of your lungs.
The problem with some air pollution is that particles are too tiny to be trapped and cleaned out by your mucous and cilia. The tiny pollution particles drift all the way to the tips of your lungs (alveoli) and can cause permanent lung damage.
Indoor air contaminants:
- Carbon dioxide (CO2), tobacco smoke, perfume, body odours — from building occupants.
- Dust, fibreglass, asbestos, gases, including formaldehyde — from building materials.
- Toxic vapours, volatile organic compounds (VOCs) — from workplace cleansers, solvents, pesticides, disinfectants, glues.
- Gases, vapours, odours — off-gas emissions from furniture, carpets, and paints.
- Dust mites — from carpets, fabric, foam chair cushions.
- Microbial contaminants, fungi, moulds, bacteria, — from damp areas, stagnant water and condensate pans.
- Ozone — from photocopiers, electric motors, electrostatic air cleaners.
Results:
- dryness and irritation of the eyes, nose, throat, and skin,
- headache,
- fatigue,
- shortness of breath,
- hypersensitivity and allergies,
- sinus congestion,
- coughing and sneezing,
- dizziness, and/or
- nausea.
- Keep your home clean and dust-free.
- Don’t let anyone smoke in your home.
- Minimize the use of potential contaminants by finding substitutes or eliminating them altogether.
- Clean (or report) any spills or leaks.
- If working in a contaminant-producing area, ensure contaminant sources are contained (e.g. work under fume hoods, clean up spills, keep doors and windows closed).
- Take your shoes off when you enter the house. The soil outside your home can contain a number of substances you do not want inside
Indoor air quality is a vital component of your home’s heating and cooling system. After all, you’ve probably heard about the impact air quality can have on your health, especially on those who suffer from allergies. And who hasn’t experienced the physical discomfort of breathing stale of stagnant air? But better indoor air quality also has another benefit. It makes your home a more comfortable, welcome place. You feel warmer in the winter. And cooler in the summer. Which means you won’t be cranking up the furnace of A.C. as much. In fact, you may be able to save up to eighteen percent on your energy bills, season after season. An air Filtration System goes a long way towards cleaning air you breathe by minimizing particles in the air. Your lungs will appreciate the cleaner air, of course. And so will your heating and cooling system. An air Filtration system can keep furnace and central air systems operating at peak efficiency, which could cut down on repair bills later on.
Individuals can take action at the local level to reduce energy use at home, on the road, at work, and at play. They can also take advantage of a number of tips (with interesting facts) and other resources to help them reduce the amount of air pollution that they create.
Community action is also important. Organizing outreach activities to promote greater community involvement is a very effective way of achieving progress. Communities, along with their municipal governments, can become involved in planning more sustainable forms of landuse and transportation as well as developing community social marketing programs to promote necessary changes.
Industry can also do its part, by adopting pollution prevention measures, pollution control technology, improving energy efficiency, switching to renewable sources, and using cleaner fuels. Many companies are improving their green image to attract growing numbers of environmentally conscious consumers.
Clean, safe air is essential in protecting the health of our people and our communities. As part of its commitment to clean up Ontario’s air, the McGuinty government has posted two new regulations that require industry to reduce their emissions of harmful air pollutants. Both of these regulations achieve commitments made in the government’s Five-Point Action Plan for Cleaner Air, announced on June 21, 2004.
Regulation 194/05 Industry Emissions – Nitrogen Oxides and Sulphur Dioxide addresses points one and two of the five-point action plan:
- Applying tough nitrogen oxides (NOx) and sulphur dioxide (SO2) limits — two of the most significant smog-causing pollutants — to more industrial sectors than ever before.
- Making the NOx and SO2 limits even stricter in future years.
Regulation 419/05 Air Pollution – Local Air Quality with better provincial standards for harmful air pollutants addresses points three to five of the plan:
- Setting new air standards, in some cases for the first time, for many harmful pollutants, including carcinogens and toxins that could pose a threat to human health.
- Achieving a better picture of industrial emissions through updated technology.
- Introducing a faster, risk-based approach to implementing new air standards.
According to Science magazine, exposure to air pollution affects “death rates, hospitalizations and medical visits, complications of asthma and bronchitis, days of work lost, restricted-activity days, and a variety of measures of lung damage.” A World Health Organization study of France, Switzerland and Austria found that their health costs due to traffic pollution amounted to approximately 1.7 percent of GDP, dramatically more than the cost of treating injuries from traffic accidents.
Source: CNN.com “Traffic pollution ‘kills thousands every year’ “ September 1, 2000
In Canada, the province of Ontario estimates that air pollution costs its 12 million residents at least $1 billion annually in hospital admissions, emergency room visits and worker absenteeism.
Source: Ontario Medical Association, “The Illness Costs of Air Pollution Ontario” June 2000
And the World Bank reports that in China – home to some of the most polluted air in the world – the deaths and illnesses of urban residents due to air pollution cost an estimated 5 percent of GDP.
We make choices every day that can help reduce air pollution. Below are a few ideas that you can take to help clean our air.
At Home
- Conserve energy – turn off appliances and lights when you leave the room.
- Recycle paper, plastic, glass bottles, cardboard, and aluminum cans. (This conserves energy and reduces production emissions.)
- Keep woodstoves and fireplaces well maintained. You should also consider replacing old wood stoves with EPA-certified models.
- Plant deciduous trees in locations around your home to provide shade in the summer, but to allow light in the winter.
- Buy green electricity-produced by low-or even zero-pollution facilities.
- Connect your outdoor lights to a timer or use solar lighting.
- Wash clothes with warm or cold water instead of hot.
- Lower the thermostat on your water heater to 120F.
- Use low-VOC or water-based paints, stains, finishes, and paint strippers.
- Test your home for radon-a dangerous, radioactive gas that is odorless and tasteless. If the test shows elevated levels of radon, the problem can be fixed cost effectively.
- Choose not to smoke in your home, especially if you have children. If you or your visitors must smoke, then smoke outside.
Buy Smart
- Buy ENERGY STAR products, including energy efficient lighting and appliances. They are environmentally friendly products. For more information,
- Choose efficient, low-polluting models of vehicles.
- Choose products that have less packaging and are reusable.
- Shop with a canvas bag instead of using paper and plastic bags.
- Buy rechargeable batteries for devices used frequently.
Drive Wise
Plan your trips. Save gasoline and reduce air pollution.
- Keep tires properly inflated and aligned.
- In the summertime, fill gas tank during cooler evening hours to cut down on evaporation. Avoid spilling gas and don’t “top off” the tank. Replace gas tank cap tightly.
- Avoid waiting in long drive-thru lines, for example, at fast-food restaurants or banks. Park your car and go in.
- When possible, use public transportation, walk, or ride a bike.
- Get regular engine tune ups and car maintenance checks (especially for the spark plugs).
- Use an energy-conserving (EC) grade motor oil.
- Ask your employer to consider flexible work schedules or telecommuting.
- Report smoking vehicles to your local air agency.
- Join a carpool or vanpool to get to work.
For Your Health
- Check daily air quality forecasts, which tell how clean or polluted your air is, and the associated health concerns.
- Remove indoor asthma triggers from your home and avoid outdoor triggers in order to effectively control your asthma. Visit www.epa.gov/asthma to learn more about asthma triggers and ways to avoid them.
- Minimize your sun exposure. Wear sun block and UV protection sunglasses. To find out about current forecasts of UV where you live, go to www.epa.gov/sunwise/uvindex.html.
Pindar (c. 522-438 B.C.), Olympian Odes
The human body is 70% water, the brain is 85% water, blood is 90% water… the human liver, one of our most vital organs is 96% water! It’s a logical assumption that the quality of the water we drink will have a very significant effect on our overall health.
Hydration Tips
- Drink at least eight 8-ounce servings of water each day. The more active you are, the more water you need to replenish lost fluids.
- Don’t wait until you’re thirsty to drink water. By the time you feel thirsty, you have probably already lost two or more cups of your total body water composition.
- Drink plenty of water throughout the day. Convenience is a must, so carry a bottle of water with you as you commute to work, run errands or enjoy a day at the beach. While at work, keep a bottle of water on your desk, or visit the office water cooler and take a water break rather than a coffee break.
- Don’t substitute beverages with alcohol or caffeine for water. Caffeine and alcohol act as diuretic beverages and can cause you to lose water through increased urination.
- Once you start exercising, drink water throughout your workout. Keep a bottle of water with you and take frequent water breaks.
- Don’t underestimate the amount of fluids lost from perspiration. Following a workout, you need to drink two cups of water for each pound lost.
- Start and end your day with water. Your body loses water while you sleep, so drink a serving before bed and again when you wake up.
- Common colds and the flu frequently lead to dehydration. Keep a large bottle of water next to your bed so you can sip it throughout the day without having to get up.
- Cool water – not carbonated beverages or sports drinks – is the best fluid for keeping hydrated when it’s warm outside. Cool water is absorbed much more quickly than warm fluids and may help to cool off your overheated body. If you’re going to be away from home or outdoors, make sure you keep a bottle of water close by.
- Make sure your children drink enough water. . Children need water to balance their intake of other beverages – especially during activities. Packing bottled water in a child’s lunch instead of juice or regular soda can also help prevent childhood obesity.
When first using softened water for household cleaning chores, it is best to use as little soap as possible. If necessary, the homeowner can gradually increase the quantity to produce the desired results.
The habit of using far too much soap is not easily broken. Therefore, anyone contacting the customer should stress the coffee measure as being adequate rather than the cup or more that may have been necessary prior to the installation of a water softener.
Neither hard nor soft water should be used with a steam iron. Demineralized or distilled water alone is acceptable for use over a period of time.
Remember, that the softening of water does not remove the minerals.
In any case, the instructions of the dishwasher manufacturer should be followed. However, these may be supplemented with the following general considerations.
Avoid the use of sudsing soaps or synthetic detergents as the suds which develop can “muffle” the spray action used in most dishwashers to provide scrubbing action. A number of washing compounds are on the market which have been specifically formulated for dishwashers. Included are such brand names as Calgonite and Electro-Sol. These materials do not generally contain any soap – they depend upon the presence of greases and oils on the dishes to produce a cleaning solution.
Even with the use of soft water, spotting of dishes is sometimes reported. In some cases, the cause may be traced to the use of too much washing compound or the use of the wrong material, as outlined above. In other cases, the dishwasher is loaded too heavily and improper cleaning occurs.
Another common cause of spotting is too rapid drying. Ideally, the dishes should first be allowed to drain completely in a humid atmosphere so that the water with its minerals runs off, rather than evaporates. In some cases, the water temperature is too high and the water evaporates rather than drains. In other cases, the dishwasher lid opens at the end of the cycle and allows cool air to contact the utensils. This, too, results in rapid evaporation.
Depending upon the construction of the dishwasher, it may be possible to adjust one or both of these factors to improve the results significantly.
Iron stains can be removed from most washable fabrics with oxalic acid, provided certain precautions are taken. Oxalic acid can be obtained at most drug stores.
A solution of the oxalic acid may be made up by simply dissolving the crystals in water. The solubility of the acid is only about 10% so, it cannot be made too strong. The solution should be prepared in an enamelled or plastic container, never in a bare metal pail.
A small amount of the solution should be tried on the inside of a hem or other inconspicuous location as the acid may bleach certain dyes. If, it is apparent that no bleaching has occurred, the entire garment may be repeatedly dipped in the solution. The dipping should continue until the iron stains are gone. Allowing the garment to soak in the acid solution is not recommended.
After the stains have disappeared, the garment should be thoroughly rinsed in several changes of fresh water. To be sure that any remaining acid has been neutralized, the garment should be immediately laundered in the normal manner with the regular amount of soap. The alkalinity in the soap will eliminate the last traces of acidity.
The acid solution may be poured down the drain but it should be followed with a thorough flushing with fresh water.
1. First, determine the sodium content of the natural water. Multiply the water’s sodium content in grains per gallon expressed as calcium carbonate, by 7.866. This will give you the sodium content of the water in milligrams per litre of water.
2. Next determine the additional sodium content of water as a result of ion exchange softening. Here multiply the total hardness of the water in grains per gallon, expressed as calcium carbonate, by 7.866.
3. A simple addition of the results of both steps 1 & 2 will give the sodium content of the softened water in milligrams of sodium per litre. One litre (slightly more than a quart) is commonly accepted as normal water consumption.
Actually, the amount of sodium present in softened water is small when compared to the sodium present in foods.
Much depends on the strictness of the diet itself.
Where the patient is on an extremely restrictive diet, he should drink neither hard nor softened water. Under these conditions, he should have demineralized water, distilled water or water known to be free of sodium for drinking and for the cooking of his foods. Such patients are commonly hospitalized.
In establishing a salt-free diet for patients, physicians should not overlook the fact that even hard water may contain appreciable amounts of sodium. To determine the amount a complete analysis of the water is necessary.
If the liquid capacity of the tank is known, multiply the figure by 8.33 to determine the amount of water in pounds. If the liquid capacity is unknown, multiply the capacity in cubic feet by .80 (approximately 20% of the chamber will be filled with air at all times). Now multiply this figure by 62.33 to obtain the pounds of water in the tank.
Compare the end figure (the total pounds of water) with the number of pounds of salt in the projected softener regeneration dosage. The pounds of salt should not exceed one percent of total amount of water in pounds in the tank.
The backwash and brining effluents from a softener can be discharged into a septic tank. The latter should, however, be properly installed and of adequate size to ensure satisfactory results. Proper sizing of the septic tank is highly important. While many states recommend units with a minimum of a 500 gallon capacity, even smaller units may provide adequate liquid capacity in relation to the amount of salts discharged by the softener.
Many public health authorities recognize that softener regeneration wastes can be safely discharged into septic tanks.
The Public Health Service in a “Manual of Septic Tank Practice” writes: “Waste brines from household water softener units have no adverse effect on the action of the septic tank but, may cause a slight shortening of the life of a disposal field installed in a structured clay-type soil.”
From this, it is evident that the type of soil in which the disposal field is situated must be taken into consideration.
High concentrations of sodium salts deposited in certain types of soil over a period of time would result in loss of permeability. When this occurs, the soil cannot absorb and transmit the waste water. As a result, the absorption capacity of the drain field gradually decreases.
On the other hand, the calcium and magnesium ions discharged by the softener may tend to open up the soil, reversing the clogging action of the sodium salts.
To date, there are no reports on file of the failure of a distribution field to absorb the sodium salts due to softener regeneration wastes.
In the event a serious doubt exists regarding the effect of the sodium wastes on the distribution field, the brine could be discharged into a dry well. Generally, a dry well (a hole filled with course gravel) will serve for many years. However, the use of a dry well should be necessary only in extremely few instances.
There is some advantage in the use of fully automatic softeners where the discharge into the brine tank may be a problem. These softeners can be set to recharge frequently but, with smaller amounts of salt.
Fully automatic softeners use from 40 to 80 gallons of water per recharging. With the operation requiring about one hour, the water flow rate is low. Further, the process is normally set to occur between midnight and 5:00 a.m., a time when little or no water is being used in the house. Under these conditions, a softener places far less burden on the capacity of the septic tank than do such water-using appliances as automatic dishwashers, washing machines or toilets, etc.
Actually, removing calcium and magnesium from the water has little effect on the quality of ice prepared in the home. Here again, the reason is that softening the water does not reduce the total mineral concentration.
For example, to the extent that a softener removes sediment, iron and manganese from water, this would help to produce a least cleaner ice.
Filters, of course, can be helpful in removing iron, turbidity, tastes and odours from water used for ice-making.
The use of polyphosphates is an economical method of treating water used in typical restaurant ice-making units. The polyphosphates keep the minerals in the water dispersed and in this way minimize the cloudy appearance of ice cubes.
If fed in proper concentrations, polyphosphates also control scale formation and corrosion in the ice cube machine. Approximately 5 ppm are recommended for scale prevention and 10 ppm for both scale prevention and corrosion control.
Where the amount of hardness minerals in the water is only moderate (less than 10 gpg), it is doubtful whether the sodium concentration would be sufficient to be a serious hazard to plants.
Most house plants require specific soil conditions for healthy growth. Many thrive best in slightly acidic soils. If there is a high hardness concentration in the water being softened, the chances are good that necessarily high sodium concentration of the soft water would be harmful to plants.
For outside sprinkling purposes, the use of softened water is first and foremost, wasteful. Again, where the concentration of hardness minerals is heavy, the sodium salts replacing them would definitely retard growth and might be sufficient to kill the grass.
Yes, a water softener will remove approximately 80-90% of the radioactive substances likely to be found in water.
However, there are several important reasons for not using a softener for this purpose. (1) It will only remove radioactive materials that are cationic in nature.
(2) Further, it will achieve only partial removal (80-90%) of these contaminants. Now, depending on possible original concentrations, the removal of even 99% of radioactive materials may not make water safe to use or drink.
(3) Also, there is the problem of disposing of the radioactive ions into the sewage system. This would be a difficult, if not, an impossible operation.
Discharge of radioactive ions into the sewage system is not recommended because of the disposal dangers. Burial of the contaminated softener after use is also not considered acceptable. Units utilized for removal of radioactive materials from water should be delivered to a central disposal site for proper handling.
In view of the limitations of softeners, they cannot be recommended for reliable, positive decontamination.
Because some radioactive substances are anionic while others are cationic, demineralization units employing strongly acidic cation resin and a strong base anion resin are necessary.
Small “throw-away” demineralizers are available for emergency needs. Such units offer the most satisfactory solution to this problem.
Soft water provides for easier maintenance of a humidifier.
When hard water is evaporated, the mineral residue consists of a hard scale which normally requires some drastic treatment (such as chipping or acid) for its removal. When soft water is used, the residue is commonly soft and can usually be removed by flushing the unit with water or by going over the surface with a brush.
A point to remember: Softening water does not reduce the total amount of minerals present; ion exchange softening merely converts the calcium and magnesium minerals to sodium minerals.
The humidifier most common in homes has an open pan, a small tube connected to a water source and float valve. When water evaporates, the float valve opens to permit makeup water to flow into the pan.
Sooner or later, this type of unit fills with minerals deposited by the inflowing water.
If soft water is used in the humidifier, periodic flushing with fresh soft water will keep the mineral concentration down and, the unit will operate satisfactorily.
Yes, soft water is satisfactory for most tropical fish. According to several authorities both fully soft water and municipally softened water would have no undesirable or toxic effect for use in an aquarium.
When making the change from hard to soft water, it is necessary to make the substitution on a gradual stepwise basis of new water for old. This follows the basic pattern in regard to any change in the environment for tropical fish. This applies to temperature, pH of water, food as well as to hardness. Drastic changes, of course, would constitute a shock to the delicate systems of such fish and would result in fatalities.
Preferably replace about one-fourth of the aquarium water at weekly intervals with soft water. Eventually, the aquarium will have a supply consisting of essentially soft water and the fish will suffer no ill effects as a result of the change.
No. Primarily the human body gains the minerals necessary to good health through eating foods not through drinking water.
The body may absorb or use the minerals in water but, in most cases, the amount would not be significant.
In order for a person to obtain significant minerals from water, it would be necessary to drink many gallons daily. In general, neither a water with a high mineral content nor a fully softened water could be considered a significant source of minerals. In contrast, one gallon of milk provides the mineral equivalent of a bathtub full of ordinary well water.
It depends on the hardness of your water, but on average less than 3% of your sodium intake comes from drinking softened water. It is estimated that the average person consumes the equivalent of two to three teaspoons of salt a day from various sources. Assuming a daily intake of 5 grams (5000 milligrams) of sodium in food and the consumption of three quarts of water (i.e., coffee, tea, fruit juices, and drinking water), the contribution of sodium (Na+) in the water from the home water softening process is minimal compared to the total daily intake of many sodium-rich foods. The formula for calculating the amount of additional sodium follows: mg of Na / quart of softened water = grains of hardness X 7.5 mg Na / grain of hardness.
Both do the same job. They replace calcium and magnesium on the softener resin during the regeneration process. When you use sodium chloride, sodium will be added to the soft water during use and when you use potassium chloride, potassium will be added to the soft water. People whose physicians have advised them to eliminate sources of sodium from their drinking water normally use potassium chloride. In some people who have kidney or other renal problems, potassium can aggravate those problems. Most healthy people(>97%) can use sodium chloride without trouble and sodium chloride is less expensive. If you have any questions, consult your physician.
No, to soften water, you need a water softener. The salt used in the brine tank of a water softener does not directly soften the water, but is used to regenerate the resin beads in your water softener. These actually soften the water from your well by removing the hard water ions, calcium, magnesium and iron.
Some of the products contain additives (primarily to keep it from getting hard or to iodize it) and others do not. Please refer to the product packaging or request a technical data sheet.
As with food considerations, water softening salts are not intended for human or animal feeding. The particle size is inappropriate for small animals. In addition, water softening salt may have additives that are inappropriate for animal feeds.
Only solar salt would be recommended. Optimum sizing of salt is ‘medium’ in gradation. Most solar salt used for water softening is coarse or extra coarse, which is larger in gradation.
Loosen any encrusted salt that may be adhering to the perimeter of the salt keeper, making sure that any large pieces are broken up. Distribute the salt evenly across the salt keeper. Make sure water level is appropriate for optimum operation.
The water level should be set according to your owners manual or at your water conditioning technician’s recommendation. The salt level should be maintained a minimum of 3 to 4 inches above the water level, unless otherwise directed by the owners manual or water conditioner technician.
Generally speaking, no; however, certain water softeners are designed for specific water softener products and may not function as well using alternative products. For example, use of rock salt in a cabinet model softener is not recommended because this type of softener is not easily cleaned and rock salt leaves a residue of insoluble matter. It can also bridge. Mixing of coarse and fine products (for example pellets and rock salt) is not recommended as bridging could also result from this practice. It is recommended that you allow your unit to go empty (or nearly empty) of one type of salt before adding another to avoid these problems.
Although water softener pellets may be made from food grade salt, the pellet press process, itself, does not meet the criteria required to call the finished pellets “food grade”. Therefore, direct application of pellets in food processing is not recommended. Other water softening salt products like solar salt, rock salt and brine blocks are not recommended for food application for the same reason.
Yes, from a physical standpoint. Cube salt runs uniformly thick at about 1/4″ and is varied in length and width. Pellets are pillow or cough drop shaped and may vary in thickness. From the standpoint of chemical purity and functionality, there is little difference to be observed, although cubes may be slightly more durable.
Salt lowers the freezing point of water making it possible to achieve below freezing temperature when applied to ice. Any type of salt, including table salt, may be used for this purpose. Since the salt will eventually melt the ice, coarse salt would be preferred because it dissolves more slowly. Rock or solar water softening salt tends to be coarse and will work well for this purpose. Pellets and Sun Gems® are too coarse and should not be used.
Yes. Evaporated salt ranges from 99.7 to 99.99% pure sodium chloride. Solar salt is typically 99.6 to 99.8% sodium chloride. Rock salt used for water conditioning may run from 95 to more than 98.5% sodium chloride, depending on the source.
Direct discharge of either sodium or potassium chloride brine should be avoided. Brine alters the osmotic pressure that grasses (plants) rely upon to regulate water needs. Imbalance in water supply will result in browning and eventually destruction of the grass. A diluted brine ratio of 20 parts water to 1 part brine may be used.
Studies performed by the Water Quality Association indicate that a properly placed septic tank with an adequate septic field is in no way impaired in operation by brine discharged from a water softener. This is primarily due to dilution factors and septic field drainage.
Over time, water-insoluble matter from salt or the water supply may accumulate in the salt holding tank. This water-insoluble matter may have the appearance of a brown or black sludge or appear oily. It is usually the result of natural mineral inclusions contained in the salt, and is generally inorganic in nature.
When seemingly all other avenues such as problems with the salt being used and/or basic mechanical malfunctions of the softener components are exhausted and the water is still not soft enough, it may be time to consider replacing the resin, or the softener. Experience has shown that, depending on water usage, most ion exchange resins last 20 to 25 years.
Potassium Chloride may be used for ion-exchange resin regeneration. It is a different type of salt that uses potassium in the ion exchange process instead of sodium. It is a more expensive product.
We do not recommend it. Note that deicing salt has a higher amount of insolubles and will require more clean-up of the brine tank. Also, the smaller particle size of deicing salt is not suitable for water softeners.
The best practice is to purchase a product that is specially designed for snow and ice removal and then to closely observe the directions for use as shown on the package. Solar Salt and Rock Salt may be used to remove snow and ice from concrete sidewalks, parking lots and asphalt surfaces. Pellets or Pellets with Iron Reducing Additive, are not recommended for this use due to their large size.
Directions for Use: Remove excess snow. Spread 1/2 to 1 cup per square yard evenly over ice and packed snow surfaces. Quantity and melting action will depend upon temperature and thickness of the ice and packed snow. Reapply as necessary. When ice and snow soften, remove slush and any excess ice melter from pavement for good concrete maintenance. Notice: All ice melting agents work by reducing the freezing point of water. This can result in more frequent freeze-thaw cycling of the melted snow and ice, which can cause spalling (surface scaling of concrete). As such, use only on properly placed, cured and sealed, air-entrained concrete. To lessen the possibility of freeze-thaw cycling and potential damage, promptly remove slush as it is formed. Do not use on porous or improperly cured concrete, concrete less than one year old, wood or other porous materials. As with any ice melting agent, particular care should be used when grass and vegetation are adjacent to the deicing surface as excess application may cause damage. Use this product at your own risk.
Unless the softener had a malfunction, or the salt has bridged or mushed and the ion-exchange resin has not been regenerated, there is nothing associated with the change that would reduce laundering capability.
It could be that the salt had too little residence time, i.e. the salt was dumped into the salt keeper and the softener regeneration cycle initiated immediately. It could also be the result of a softener malfunction or possibly salt bridging or mushing which reduces or eliminates brine formation.
This question often accompanies a switch from one type of water softening salt to another, e.g. going from pellets to solar. What you are seeing perhaps is related to a difference in bulk density between the two products. The closer the crystals pack together, the less volume they occupy. This would give rise to the perception that the salt is dissolving too fast. In addition, if the initial water level in your unit was set to use pellets, it may be too high to use solar salt (i.e. too much water in the tank dissolves more salt) and may need to be lowered. Follow your softener manufacturer’s instructions.
In reality, salt can only dissolve to the extent that it produces a saturated brine (26.4% by weight). It doesn’t matter what salt is used. When the brine is saturated with respect to salt, no more can dissolve. Therefore, even though appearances may suggest otherwise, salt usage is the same regardless of the salt product type or form that is used.
Check the salt at the water level to see if a solid mass has developed (called a “bridge”), or if fine “mushy” salt is lying at the bottom of the tank (called mushing). If a bridge, carefully break up the mass to allow it to drop into the water below. If mushing, remove the good pellets, scoop out the “mushed” salt, and reload the good pellets.
If the salt keeper was empty at the time of fresh salt addition, check the water level in the tank. If lower than normal, the float may be stuck in the internal side column. Remove the cover and check the mechanism to determine if it is working freely. If not, call the service department of the water softener manufacturer and arrange for a service call.
The smell of rotten eggs is generally associated with hydrogen sulfide gas that may be present in the water supply. Salt does not remove this odor or the gas. You must take other steps to remove the gas.
Normally, blocks are used in specially designed salt holding tanks. For proper operation, the water level in the holding tank is raised to keep the blocks submerged for maximum brine formation. If you want to switch to salt blocks you may have to reset the water level in the salt keeper.
Pellets may be made from compacted evaporated salt or compacted solar salt. The latter product is usually slightly darker in color.
No, solar salt is a natural product made by evaporating seawater. It is harvested much like an agricultural crop and consequently may contain minute inclusions such as earth, small pebbles, and other naturally occurring materials.
Since these inclusions are of a different density than the brine in the bottom of the salt keeper, they are generally left behind in the salt keeper. Lighter density materials, should they accompany the salt brine during regenerations, are usually flushed from the resin during the rinse cycle which follows regeneration.
The type of salt best suited to a particular softener will vary in accord with softener design. Usually, cabinet-style self-contained softeners require salt that is low in water-insoluble matter, while side-by-side units with separate salt holding tanks are easier to clean and therefore allow more flexibility in choosing a salt product. Usually, however, we recommend salt in Pellets which are of a high salt purity.
Iron removal through the use of an iron reducing additive is limited to water containing 2 ppm (parts per million) iron or less. With iron levels that are higher, a strong single-dose cleanser (usually available through water conditioning dealers or hardware stores) should be used periodically. If your water has a significant level of iron, you may have to install a special iron filter.
Unless the salt product being used is high in water-insoluble matter, or there is a serious malfunction of some sort (e.g. bridging), it is usually not necessary to clean out the brine tank. Some individuals choose to allow all of the salt to dissolve in their softener unit once per year so it can be visually inspected to insure no build-up has occurred. If there is a build-up, it should be cleaned out to prevent softener malfunction. However, an annual inspection is not mandatory.
If this condition presents itself with some frequency, you may reduce the tendency by limiting the amount of salt added to the “salt-keeper” (e.g. if currently adding four 40 lb. bags, reduce to two bags).
This condition, known as “bridging” or “mushing” will require manual break up of the salt mass to facilitate brine flow. A handy person can probably accomplish this task, but alternatively, a service call may be arranged through a water conditioning dealer.
Rock salt will work in a softener; however, because of the relatively high level of water insoluble matter present in rock salt, it is recommended for use only if the consumer is willing to perform routine brine tank cleanout. For the average home softener this can be required 2 to 3 times per year.
Since solar salt contains slightly more water insoluable matter than (evaporated salt) pellets, consideration should be given to salt usage, softener cleanout frequency and softener design. If salt usage is light one could probably use the products interchangeably. If salt usage is heavy, insoluables will build up faster when using solar salt, and the need to more frequently clean the brine tank/reservoir will be increased. Brine tank cleanout can be a messy task.
Three types of salt are generally encountered in the retail store: rock salt, solar salt (crystals) and evaporated salt (pellets).
Rock salt is a naturally occurring mineral which is obtained from underground salt deposits by traditional mining methods. It exhibits a variable chemical purity, running from 98% to 99% sodium chloride. It has a water insolubles level of about 0.5% to 1.5%, the chief component of which is an impurity called calcium sulfate.
Solar salt is a natural product obtained through the evaporation of seawater or inland brine sources. It has a sodium chloride content of 99.5% or higher, and a water insolubles level of less than 0.03%. It is most commonly sold in a crystal form, but also may be sold in the form of compressed pellets or blocks.
Evaporated salt is manufactured by solution mining underground bedded salt deposits of dissolving salt to form a brine and then evaporating the moisture using energy in the form of natural gas or coal. Evaporated salt (in the form of compacted pellets, sheeted salt – called cubes, or blocks) has a sodium chloride content ranging from 99.6% to 99.99%. Water insoluble matter generally is less than 0.01%.
The more often you regenerate, the more often you’ll need to add salt. A good general rule of thumb is to check your softener once a month. To maintain consistently soft water, keep your salt level at least half-full at all times, but do not overfill.
Bridging is a condition that sometimes occurs in the brine tank when salt sticks together forming a “bridge” that prohibits it from coming into contact with the water in the tank. You can eliminate bridging by using a 100% water soluble pellet product in your brine tank.
Occasionally, if you use salt pellets or cube-style salt which are too loosely compacted, they may revert to tiny crystals of evaporated salt – similar to table salt. These crystals may bond, creating a thick mass in your brine tank. This mushing may interrupt brine production – the key element for refreshing the resin beads in your softener. Without brine, your softener can’t produce soft water.
No. Salt’s sole purpose in your water softener is to regenerate the resin beads that actually take the hardness out of your water. This exchange does not make your water taste salty or significantly increase your sodium intake.
What is TDS?
Interesting Water Facts
- In just 16 hours, U.S. water utilities produce as much potable water as the oil industry produces oil in a year.
- When we use water, we generally add contaminants to it, such as soap, food products, and chmeicals, which must be removed before the water is used again.
- Close to 3/4 of the Earth’s surface is covered with water, but less than 1% is suitable and available for drinking using conventional water treatment.
- Ice cubes float because ice is less dense than water. Water freezes in a lattice-like formation, which creates buoyancy and allows ice to float.
- Hardness in drinking water is caused by calcium and magnesium – two non-toxic, naturally occuring minerals in water. Excessive hardness makes it difficult for soap to lather, leaves spots on dishware and reduces water flow.
- Water is the original health drink. It contains no fat, no calories and no cholesterol.
- Becausee 60% of an adult’s body is water, it is essential to replenish the water you lose through breathing, perspiration and excretion. For most people, this equates to approximately 8 cups (2 liters) a day. We can consume water not only by drinking water, but also through food and other beverages.
- Through the processes of evaporation, condensation, precipitation and infiltration – the hydrologic cycle – the total amount of water on Earth remains constant. The availability of fresh drinking water, however, continues to diminish, as demand continues to increase.
SOURCE: American Water Works Association (AWWA), 2010
History of Water Quality, Purification and Filtration
Water is one of the most important aspects of life on Earth, and yet so many of us take it for granted. Without water, life on Earth would cease to exist. Plants, animals and human beings all require water. It is, in a sense, life’s battery. Water can also be detrimental, though, if not kept clean and potable.
The dichotomy of its existence…it is a life giver and also a life taker. It is for this reason that humans have been working on methods of obtaining pure water since the beginning of human existence. Archeological evidence shows that people have been attempting to purify water and give it a pleasurable taste since prehistoric times.
Early humans believed that the purity of water was determined by taste; if the water had a pleasing taste, then it was pure. As a result, the method of purifying water in ancient times was to add herbs or flowers to the water, but this was obviously not a true method of purification, nor was it enough to quell the search for one.
In 2000 B.C., Sanskrit documents about medical concerns were created called the Sus’ruta Samhita. The Sus’ruta Samhita declares that “impure water should be purified by being boiled over a fire, or being heated in the sun or by dipping a heated iron into it, or it may be purified by filtration through sand and coarse gravel and then allowed to cool.”
Inscriptions have been discovered on the walls of the tomb of Amenophis II in Thebes. The inscription depicts the Egyptian method of purifying water in which they siphoned the water through a series of wick siphons. The inscription has been dated 1450 B.C.
The Bible even has evidence of possible methods of water purification. In Exodus 15:22-27, Moses and the Israelites came upon Marah and found that the waters there were bitter. Moses was guided towards a tree and told to place the tree into the waters of Marah. Moses did as he was instructed and the waters of Marah were sweetened thereafter. While it is unclear what type of tree this was, or if any type of filtration process was used, the evidence points to at least a concern about water quality.
Another early method of filtering water was developed by the famous Greek doctor, Hippocrates, in the 3rd century B.C. Recognizing that boiling water did not remove suspended solids, Hippocrates used a cloth bag to strain the water after boiling it. This method was later called “Hippocrates’ Sleeve.”
In 1627, Sir Francis Bacon compiled 10 experiments in A Natural History of Ten Centuries. He was led to believe that water could be filtered through sand when he read about a successful experiment purifying seawater in this manner.
In the late 1600s, Lucas Antonius Portius, an Italian physician, wrote about the multiple sand filtration method. The method had 3 pairs of sand filters consisting of downward and upward flow.
Following these discoveries, sand filters and rainwater cisterns were developed. La Hire, a French scientist, proposed in 1703 that all households should have a rainwater cistern along with a sand filter. 100 years later, the first municipal water treatment plant was installed in Paisley, Scotland.
In the 20th and 21st centuries, water quality has become even more important and numerous methods of water purification and filtration have been developed.
References:
1. National Environmental Services Center: http://www.nesc.wvu.edu/ndwc/ndwc_DWH_1.html
2. “Water Quality and Treatment: A Handbook of Public Water Supply.” American Water Works Association (AWWA). 1971.
What is Water?
Water (H2O) is essential to life on Earth and more than two thirds of the planet’s surface is made up of water in its solid, gas and liquid forms – the only chemical compound on Earth that naturally exists in all three physical states.
The properties of liquid water help dissolve molecules, which enable chemical reactions to occur, yielding friendly conditions for life. Vital substances like metabolites and nutrients are able to travel through liquid water microscopically, as well as globally. Water also greatly assists enzymes in catalyzing chemicals, which is also essential for life.
Pure water freezes at 32°F (0°C) and boils at 212°F (100°C) although impurities can alter these figures. This wide temperature range as a liquid also helps preserve life on Earth since typical fluctuations in the weather do not have cataclysmic effects on the planet, which is mostly covered with water.
The human body is also mostly made up of water, and we depend on water to grow food and survive. The global modern industrial infrastructure requires water for a wide range of things, from cooling down machinery to the use of water for transportation and shipping. An uncountable number of organisms live in bodies of fresh water, seawater or brine, whether they are puddles or oceans.
Water is indeed one of Earth’s indispensable resources in creating and maintaining life.